Liner Programming Class 12 MCQs Questions with Answers
Linear Programming Class 12 MCQ Chapter 12 Question 1.
The corner points of the feasible region determined by the system of linear constraints are (0,0), (0,40), (20,40), (60,20), (60,0). The objective function is Z = 4x + 3y.
Compare the quantity in Column A and Column B
| Column A | Column B |
| Maximum of Z | 325 |
(A) The quantity in column A is greater.
(B) The quantity in column B is greater.
(C) The two quantities are equal.
(D) The relationship cannot be determined on the basis of the information supplied.
Answer:
(B) The quantity in column B is greater.
Explanation:
| Corner points | Corresponding value of Z = 4x + 3y |
| (0, 0) | 0 |
| (0, 40) | 120 |
| (20, 40) | 200 |
| (60, 20) | 300 ←Maximum |
| (60, 0) | 240 |
Hence, maximum value of Z = 300 < 325 So, the quantity in column B is greater.
![]()
MCQ Questions On Linear Programming Class 12 Question 2.
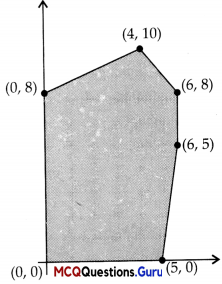
The feasible solution for a LPP is si iown in given figure. Let Z = 3x – 4y be the objective function Minimum of Z occurs at
(A) (0,0)
(B) (0,8)
(C) (5,0)
(D) (4,10)
Answer:
(B) (0,8)
Explanation:
| Corner points | Corresponding value of Z = 3x – 4y |
| (0,0) | 0 |
| (5,0) | 15 ←Maximum |
| (6,5) | -2 |
| (6,8) | -14 |
| (4, 10) | -28 |
| (0, 8) | -32 ←Minimum |
Hence, the minimum of Z occurs at (0, 8) and its minimum value is (-32).
Linear Programming MCQ Chapter 12 Class 12 Question 3.
The feasible solution for a LPP is si iown in given figure. Let Z = 3x – 4y be the obje .ctive function Minimum of Z, maximum of Z occurs at
(A) (5,0)
(B) (6,5)
(C) (6, 8)
(D) (4,10)
Answer:
(A) (5,0)
Explanation:
Maximum of Z occurs at (5, 0).
![]()
Linear Programming MCQ With Answers Pdf Question 4.
The feasible solution for a LPP is si iown in given figure. Let Z = 3x – 4y be the obje .ctive function Minimum of Z , (Maximum va lue of Z + Minimum value of Z) is equal to ……………
(A ) 13
(B) 1
(C) -13
(D) -17
Answer:
(D) -17
Explanation:
Maximum value of Z + Minimum value of Z = 15 – 32 = -17
MCQ Of Linear Programming Class 12 Question 5.
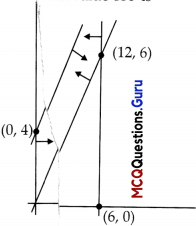
The t easible region for an LPP is shown in the given Figure. Let F = 3x – 4y be the objective function. Maximum value of F is …………..
(A) 0
(B) 8
(C) 12
(D) -18
Answer:
(C) 12
Explanation:
The feasible region as shown in the figure, has objective function F = 3x – 4y.
| Corner points | Corresponding value of F = 3x – 4y |
| (0,0) | 0 |
| (12,6) | 12 ←Maximum |
| (0,4) | -16 ←Maximum |
Hence, the maximum, value of F is 12.
MCQ On Linear Programming Class 12 Question 6.
The teasible region for an LPP is shown in the given Figure. Let F = 3x – 4y be the objective function, minimum value of F is …….
(A) 0
(B) -16
(C) 12
(D) does not exist
Answer:
(B) -16
Explanation:
Minimum value of F is -16 at (0,4).
![]()
MCQ On Linear Programming Chapter 12 Question 7.
Corner points of the feasible region for an LPP are. (0,2), (3,0), (6,0), (6,8) and (0, 5). Let F = 4x + 6y be the objective function. The minimum value of F occurs at
(A) (0, 2) only
(B) (3, 0) only
(C) the mid-point of the line segment joining the points (0, 2) and (3, 0) only
(D) any point on the line segment joining the points (0, 2) and (3, 0)
Answer:
(D) any point on the line segment joining the points (0, 2) and (3, 0)
Explanation :
Corner points Corresponding value of F = 4x + 6y
| Corner points | Corresponding value of F = 4x + 6y |
| (0,0) | 12 ←Maximum |
| (3,0) | 12 ←Maximum |
| (0,6) | 24 |
| (6, 8) | 72 ←Maximum |
| (0,5) | 30 |
Hence, minimum value of F occurs at any points on the line segment joining the points (0, 2) and (3,0).
Class 12 Maths Chapter 12 MCQ Question 8.
Corner points of the feasible region for an LPP are. (0,2), (3,0), (6,0), (6,8) and (0, 5). Let F = 4x + 6y be the objective function, Maximum of F – Minimum of F =
(A) 60
(B) 48
(C) 42
(D) 18
Answer:
(A) 60
Explanation:
Maximum of F – Minimum of F = 72 – 12 = 60
Linear Programming MCQs Chapter 12 Question 9.
Corner points of the feasible region determined by the system of linear constraints are (0, 3), (1,1) and (3, 0). Let Z = px + qy, where p, q > 0. Condition on p and q so that the minimum of Z occurs at (3,0) and (1, 1) is ……….
(A) p = 2q
(B) p = \(\frac {q}{2}\)
(C) p = 3q
(D) p = q
Answer:
(B) p = \(\frac {q}{2}\)
Explanation:
| Corner points | Corresponding value of Z = px – qy; p, q > 0 |
| (0,3) | 3q |
| (1,1) | P + q |
| (3,0) | 3p |
So, condition of p and Question so that the minimum of Z occurs at (3,0) and (1,1) is
p+q = 3p
2p = q
p = \(\frac {q}{2}\)
![]()
Assertion And Reason Based MCQs (1 Mark each)
Directions: In the following questions, A statement of Assertion (A) is followed by a statement of Reason (R). Mark the correct choice as
(A) Both A and R are true and R is the correct explanation of A
(B) Both A and R are true but R is NOT the correct explanation of A
(C) A is true but R is false
(D) A is false but R is True
Linear Programming MCQ Class 12 Chapter 12 Question 1.
Assertion (A): Feasible region is the set of points which satisfy all of the given constraints and objective function too.
Reason (R): The optimal value of the objective function is attained at the points on X-axis only.
Answer:
(C) A is true but R is false
Explanation:
The optimal value of the objective function is attained at the comer points of feasible region.
Question 2.
Assertion (A): The intermediate solutions of constraints must be checked by substituting them back into objective function.
Reason (R):
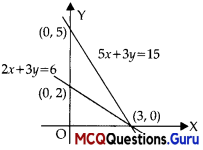
Here (0,2); (0,0) and (3,0) all are vertices of feasible region.
Answer:
(D) A is false but R is True.
Explanation:
The intermediate solutions of constraints must be checked by substituting them back into constraint equations.
![]()
Linear Programming Class 12 MCQ Questions Question 3.
Assertion (A) : For the constraints of linear optimizing function Z = x1 + x2 given by x1 + x2 ≤ 1, 3x1 + x2 ≥ 1, there is no feasible region.
Reason (R): Z = 7x + y, subject to 5x + y ≤ 5, x + y ≥ 3, x ≥ 0, y > 0. Out of the corner points of feasible region (3, 0), (\(\frac {1}{2}\), \(\frac {5}{2}\)), (7, 0) and (0,5), the maximum value of Z occurs at (7, 0).
Answer:
(B) Both A and R are true but R is NOT the correct explanation of A
Explanation:
Assertion (A) is corred. Clearly from the graph below that there is no feasible region.
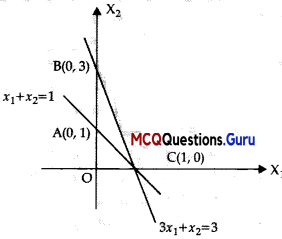
Assertion (R) is also correct.
| Corner points | Corresponding value of Z = 7x + y |
| (3,0) | 21 |
| (\(\frac {1}{2}\), \(\frac {5}{2}\)) | 6 |
| (7,0) | 49 Maximum |
| (0,5) | 5 |
Linear Programming MCQ With Answers Chapter 12 Question 4.
Assertion (A): Z = 20x1 + 20x2, subject to x1 ≥ 0, x2 ≥ 2, x1 + 2x2 ≥ 8, 3x1 + 2x2 ≥ 15, 5x1 2x2 ≥ 20. Out, of the corner points of feasible region (8, 0), (\(\frac {5}{2}\),\(\frac {15}{2}\)), (\(\frac {7}{2}\),\(\frac {9}{4}\)) and (0,10), the mini mum value of Z occurs at \(\frac {7}{2}\),\(\frac {9}{4}\).
Reason (R):
| Corner points | Z = 20x + 20y |
| (8,0) | 160 |
| (\(\frac {5}{2}\), \(\frac {15}{4}\)) | 125 |
| (\(\frac {7}{2}\), \(\frac {9}{4}\)) | 115 minimum |
| (0,10) | 200 |
Answer:
(A) Both A and R are true and R is the correct explanation of A
Explanation:
Assertion (A) ard Reason (R) both are correct and Reason (R) is the correct explanation of Assertion (A).
![]()
Question 5.
Assertion (A): For the constraintc of a LPP problem given by x1 + 2x2 ≤ 2000, x1 + x2 ≤ 1500, x2 ≤ 600 and x1, x2 ≥ 0, the points (1000, 0), (0, 500), (2, 0) lie in the positive bounded region, but point (2000, 0) does not lie in the positive bounded region.
Reason (R):
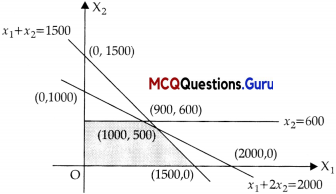
From the graph, it is clear that the point (2000, 0) is outside.
Answer:
(A) Both A and R are true and R is the correct explanation of A
Explanation:
Assertion (A) and Reason (R) both are correct, Reason (R) is the correct explanation of Assertion (A).
Question 6.
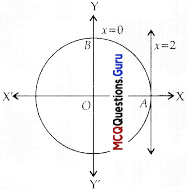
Assertion (A) : The graph of x ≤ 2 and y ≥ 2 will be situated in the first and second quadrants.
Reason (R):
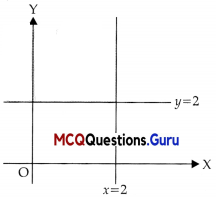
Answer:
(A) Both A and R are true and R is the correct explanation of A
Explanation:
It is clear from the graph given in the Reason (R) that Assertion (A) is true.
![]()
Case-Based MCQs
Attempt any four sub-parts from each question. Each sub-part carries 1 mark.
I. Read the following text and answer the following questions on the basis of the same:
An aeroplane can carry a maximum of 200 passengers. A profit of ₹ 1000 is made on each executive class ticket and a profit of ₹ 600 is made on each economy class ticket. The airline reserves at least 20 seats for the executive class. However, at least 4 times as many passengers prefer to travel by economy class, than by executive class. It is given that the number of executive class tickets is x and that of economy class tickets is y.

Question 1.
The maximum value of x + y is ………………
(A) 100
(B) 200
(C) 20
(D) 80
Answer:
(B) 200
Question 2.
The relation between x and y is ………..
(A) x < y (B) y > 80
(C) x ≥ 4y
(D) y ≥ 4x
Answer:
(D) y ≥ 4x
Question 3.
Which among these is not a constraint for this LPP?
(A) x ≥ 0
(B) x + y ≤ 200
(C) x ≥ 80
(D) 4x – y ≤ 0
Answer:
(C) x ≥ 80
![]()
Question 4.
The profit when x = 20 and y = 80 is ……….
(A) ₹60,000
(B) ₹68,000
(C) ₹64,000
(D) ₹1,36,000
Answer:
(B) ₹68,000
Question 5.
The maximum profit is ₹ …………..
(A) 1,36,000
(B) 1,28,000
(C) 68,000
(D) 1,20,000
Answer:
(A) 1,36,000
Explanation:
Objective function :
Maximise Z = 1000x + 600y
Constraints:
x + y ≥ 200
y ≥ 20, x > 0
y ≥ 4x
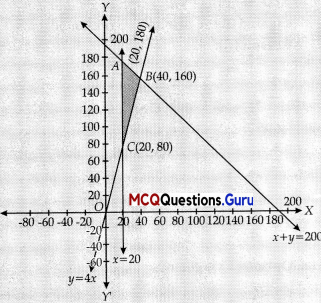
The corner points are 1(20, 180), 8(40, 160), C(20,80)
Evaluating the objective function
Z = 1,000x + 600y at A, B and C
At A(20,180), Z = 1,000 x 20 + 600 x 180
= 20,000 + 1,08,000
= ₹1,28,000
At B(40,160), Z = 1,000 x 40 + 600 x 160
= 40,000 + 96,000
= ₹1,36,000 (max.)
At C(20, 80), Z = 1000 x 20 + 600 x 80
= 20,000 + 48,000
= ₹68,000
or
Z is maximum, when x = 40, y = 160.
or
40 tickets of executive class and 160 tickets of economy class should be sold to get the maximum profit of ₹1,36,000.
![]()
II. Read the following text and answer the following questions on the basis of the same:
A dealer in rural area wishes to purchase a number of sewing machines. He has only ₹5,760 to invest and has space for at most 20 items for storage. An electronic sewing machine cost him ₹360 and a manually operated sewing machine ₹240. He can sell an electronic sewing machine at a profit of ₹22 and a manually operated machine at a profit of ₹18. Assume that the electronic sewing machines he can sell is x and that of manually operated machines is y.
Question 1.
The objective function is …………..
(A) Maximize Z = 360x + 240y
(B) Maximize Z = 22x + 18y
(C) Minimize Z = 360x + 240y
(D) Minimize Z = 22x + 18y
Answer:
(B) Maximize Z = 22x + 18y
Question 2.
The maximum value of x + y is ………….
(A) 5760
(B) 18
(C) 22
(D) 20
Answer:
(D) 20
Question 3.
Which of the following is not a constraint ₹
(A) x + y ≥ 20
(B) 360x + 240y ≤ 5,760
(C) x ≥ 0
(D) y ≥ 0
Answer:
(A) x + y ≥ 20
Question 4.
The profit is maximum when (x, y) =
(A) (5,15)
(B) (8,12)
(C) (12,8)
(D) (15,5)
Answer:
(B) (8,12)
![]()
Question 5.
The maximum profit is …………..
(A) 5,760
(B) 392
(C) 362
(D) 290
Answer:
(B) 392
Explanation:
Objective function :
Maximize Z = 22x + 18y
Constraints :
x + y ≤ 20
360x + 240y ≤ 5,760
Or
3x + 2y ≤ 48
x ≥ 0, y ≤ 0
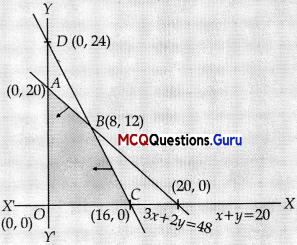
Vertices of feasible region are :
A(0, 20), B(8,12), C(16, 0) & O(0,0)
P(A) = 360, P(B) = 392, P(C) = 352
∴ For Maximum P, Electronic machines = 8, and Manual machines = 12. Max. profit ₹392
III. Read the following text and answer the following questions on the basis of the same:
A fruit grower can use two types of fertilizer in his garden, brand P and brand Q. The amounts (in kg) of nitrogen, phosphoric acid, potash, and chlorine in a bag of each brand are given in the table. Tests indicate that the garden needs at least 240 kg of phosphoric acid, at least 270 kg of potash and at most 310 kg of chlorine.
| kg per bag | kg per bag | kg per bag |
| Brand P | Brand Q | |
| Nitrogen | 3 | 3.5 |
| Phosphoric acid | 1 | 2 |
| Potash | 3 | 1.5 |
| Chlorine | 1.5 | 2 |
Question 1.
The Objective function to minimise the amount of nitogen added to garden?
(A) Maximise Z = 3x + 4y
(B) Minimise Z = 3x + 3.5y
(C) Maximise Z = 4x + 3.5y
(D) Minimise Z = 3x + 4y
Answer:
(B) Minimise Z = 3x + 3.5y
![]()
Question 2.
If the grower wants to minimise the amount of nitrogen added to the garden, how many bags of brand P should be used?
(A) 40
(B) 50
(C) 100
(D) 60
Answer:
(A) 40
Question 3.
If the grower wants to minimise the amount of nitrogen added to the garden, how many bags of brand Q should be used?
(A) 40
(B) 50
(C) 100
(D) 60
Answer:
(C) 100
Question 4.
What is the minimum amount of nitrogen added in the garden?
(A) 595 kg
(B) 550 kg
(C) 400 kg
(D) 470 kg
Answer:
(D) 470 kg
![]()
Question 5.
What is the total number of bags used by fruit grower to minimise the amount of nitrogen?
(A) 160
(B) 190
(C) 140
(D) 130
Answer:
(C) 140
Explanation:
Let the fruit grower use x bags of brand P and y bags of brand Q.
The problem can be formulated as follows:
Minimise Z = 3x + 3.5y ……(i)
Subject to the constraings,
x + 2y ≥ 240 ……….(ii)
x + 0.5y ≥ 90 ………(iii)
1.5x + 2y ≤ 310 ……(iv)
x,y ≥ 0 …..(v)
The feasible region determined by the system of constraints is as follows:
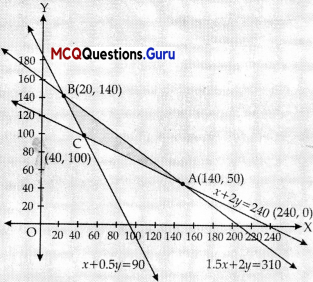
The corner points are A(240, 50), B(20, 140), and C(40,100)
| Corner points | Z = 3x + 3.5y | |
| A(140,50) | 595 | |
| B(20,140) | 550 | |
| C(40,100) | 470 | ←Maximum |
The minimum value of Z is 470 at (40,100).
Thus, 40 bags of brand P and 100 bags of brand Q should be added to the garden to minimise the amount of nitrogen.
The minimum amount of nitrogen added to the garden is 470 kg.