Electrochemistry Class 12 MCQs Questions with Answers
Electrochemistry MCQ Chemistry Chapter 3 Question 1.
Debye-Huckel Onsager equation for strong electrolytes: \(\wedge=\wedge_{0}-\mathrm{A} \sqrt{\mathrm{C}}\) Which of the following equality holds?
(A) \(\wedge=\wedge_{\mathrm{o}} \text { as } C \longrightarrow \sqrt{A}\)
(B) \(\wedge=\wedge_{0} \text { as } \mathrm{C} \longrightarrow \infty\)
(C) \(\wedge=\wedge_{\mathrm{o}} \text { as } \mathrm{C} \longrightarrow 0\)
(D) \(\wedge=\wedge_{o} \text { as } \mathrm{C} \longrightarrow 1\)
Answer:
(B) \(\wedge=\wedge_{0} \text { as } \mathrm{C} \longrightarrow \infty\)
Explanation:
When c → ∞
Then \(\wedge=\wedge_{o}\)
Electrochemistry Class 12 MCQ Chapter 3 Question 2.
Which of the following option will be the limiting molar conductivity of CH3COOH if the limiting molar conductivity of CH3COONa is 91 Scm2 mol-1? Limiting molar conductivity for individual ions are given in the following table.
| Ions | limiting molar conductivity / Scm2mol-1 |
| 1.H+ | 349.6 |
| 2.Na+ | 50.1 |
| 3. K+ | 73.5 |
| 4. OH | 199.1 |
(A) 350 Scm2 mol-1
(B) 375.3 Scm2 mol-1
(C) 390.5 Scm2 mol-1
(D) 340.4 Scm2 mol -1
Answer:
(C) 390.5 Scm2 mol-1
Explanation:
The limiting molar conductivity (A$ for strong and weak electrolyte can be determined by using Kohlrausch’s law which states that “the limiting molar conductivity of an electrolyte can be represented as the sum of the individual contributions of the anion and cation of the electrolyte.”
![]()
\(\wedge\) CH3COONa = \(\wedge\) CH3COO“ + ANS1
91 Scm2 mol-1 = \(\wedge\) CH3COO + 50.1 Scm2 mol-1
=> \(\wedge\)CH3COO” = 40.9 Scm2 mol-1
For acetic acid,
\(\wedge\) CH3COOH = \(\wedge\)CH3COO– + \(\wedge\)H+
= 40.9 Scm2 mol-1 + 349.6 Scm2 mol-1 = 390.5 Scm2 mol-1
MCQ On Electrochemistry Chemistry Chapter 3 Question 3.
Which of the statements about solutions of electrolytes is not correct ?
(A) Conductivity of solution depends upon size of ions.
(B) Conductivity depends upon viscosity of solution.
(C) Conductivity does not depend upon solvation of ions present in solution.
(D) Conductivity of solution increases with temperature.
Answer:
(B) Conductivity depends upon viscosity of solution.
Explanation:
Conductivity depends upon solvation of ions present in solution. Greater the solvation of ions of an electrolyte, lesser will be the electrical conductivity of the solution.
Electrochemistry MCQ Questions Chapter 3 Question 4.
When 0.1 mol COCl3(NH3)5 is treated with excess of AgNO3, 0.2 mol of AgCl are obtained. The conductivity of solution will correspond to
(A) 1 : 3 electrolyte
(B) 1 : 2 electrolyte
(C) 1 : 1 electrolyte
(D) 3 : 1 electrolyte
Answer:
(B) 1:2 electrolyte
Explanation:
When 0.L mole of COCl3(NH3)5 was reacted with excess of Ag.NO3, we gel 0.2 moles of Agt’l. So, there are two chloride ions that are free and not part of the complex. The formula for 1 complex has to be [CO(NH3)5Cl]Cl2 [CO(NH3)Cl]Cl2 → [CO(.NH3)5Cl]2+ + 2Cl– Therefore, the conductivity of the solution will be 1: 2 electrolyte.
![]()
MCQ Of Electrochemistry Class 12 Chapter 3 Question 5.
The cell constant of a conductivity cell
(A) Changes with change of electrolyte.
(B) Changes with change of concentration of electrolyte.
(C) Changes with temperature of electrolyte.
(D) Remains constant for a cell.
Answer:
(D) Remains constant for a cell.
Explanation:
The cell constant of a conductivity cell remains constant for a cell.
Electrochemistry MCQ Class 12 Chemistry Question 6.
\(\Lambda^{0} m\left[\mathrm{NH}_{4} \mathrm{OH}\right]\) is equal to …………..
(A) \(\Lambda_{\mathrm{m}\left[\mathrm{NH}_{4} \mathrm{OH}\right]}^{0}+\Lambda_{\mathrm{m}\left[\mathrm{NH}_{4} \mathrm{Cl}\right]}^{0}-\Lambda_{[\mathrm{HCl}]}^{0}\)
(B) \(\Lambda_{\mathrm{m}\left[\mathrm{NH}_{4} \mathrm{Cl}\right]}^{0}+\Lambda_{\mathrm{m}[\mathrm{Na} \mathrm{OH}]}^{0}-\Lambda_{[\mathrm{NaCl}]}^{0}\)
(C) \(\Lambda_{\mathrm{m}\left[\mathrm{NH}_{4} \mathrm{Cl}\right]}^{0}+\Lambda_{\mathrm{m}[\mathrm{NaCl}]}^{0}-\Lambda_{[\mathrm{NaOH}]}^{0}\)
(D)\(\Lambda_{\mathrm{m}[\mathrm{NaOH}]}^{0}+\Lambda_{\mathrm{m}[\mathrm{NaCl}}^{0}-\Lambda_{\left[\mathrm{NH}_{\mathrm{C}} \mathrm{Cl}\right]}^{0}\)
Answer:
(B) \(\Lambda_{\mathrm{m}\left[\mathrm{NH}_{4} \mathrm{Cl}\right]}^{0}+\Lambda_{\mathrm{m}[\mathrm{Na} \mathrm{OH}]}^{0}-\Lambda_{[\mathrm{NaCl}]}^{0}\)
Explanation:
NH4Cl NH4Cl ⇌ NH+4Cl–
NaCl ⇌ Na+ + Cl– (ii)
NaOH ⇌ Na+ OH– (iii)
NH4OH ⇌ NH+4 + 0H– (iv)
To get equation (iv)
\(\mathrm{A}_{\mathrm{m}}^{\circ}\left(\mathrm{NH}_{4} \mathrm{Cl}\right)+(\mathrm{NaOH})^{-} \Lambda_{\mathrm{m}}^{\circ}(\mathrm{NaCl})=\Lambda^{\circ}{ }_{\mathrm{m}}\left(\mathrm{NH}_{4} \mathrm{OH}\right)\)
Class 12 Chemistry Chapter 3 MCQ Chemistry Question 7.
In the electrolysis of aqueous sodium chloride solution which of the half cell reaction will occur at anode?
(A) Na+ (aq) + e– → Na(s); EΘcell= 2.71 V
(B) 2H2O(l) → O2(g) + 4H+(aq) + 4e– EΘcell = 1.23V
(C) H+ (aq) + e– → \(\frac {1}{2}\) H2(g); EΘcell = 0.00 V
(D)C– → \(\frac {1}{2}\) Cl2(g) + e; EΘcell = 1.36V
Answer:
(B) 2H2O(l) → O2(g) + 4H+(aq) + 4e– EΘcell = 1.23V
Explanation:
During electrolysis
NaCl →Na+ + Cl–
H2O →H+ + OH–
Na– + e– → Na(EΘcell = – 2.71V)
H+ + e– \(\frac {1}{2}\) 4H2 (EΘcell = 0.00V)
Atcathode,
H2O + e– → \(\frac {1}{2}\)H2 + OH–
At anode, two reactions are possible.
Cl– → \(\frac {1}{2}\)Cl2 + e–; EΘcell = 1.36 V
2H2O → O2 + 4H + 4e–; EΘcell = 1.23 V
![]()
MCQ On Electrochemistry Class 12 With Answers Question 8.
Which of the following statement is corred?
(A) Ecell and ∆rG of cell reaction both extensive properhes.
(B) Ecell and ∆rG of cell reaction both intensive properties.
(C) Ecell is an intensive property while ∆rG of cell reaction is an extensive property.
(D) Ecell is an extensive property while ∆rG of cell reaction is an intensive property.
Answer:
(C) Ecell is an intensive property while ∆rG of cell reaction is an extensive property.
Explanation:
Eu is an intensive property and it does not depend upon number of partides but ∆rG of the cell reaction is an extensive property because this depends upon number of particles.
Electrochemistry MCQs Chemistry Chapter 3 Question 9.
An elecftochemical cell behaves like an electrolytic cell when
(A) Ecell = Eexternal
(B) Eexternal = 0
(C) Eexternal > Ecell
(D) Eexternal < Ecell
Answer:
(C) Eexternal > Ecell
Explanation:
If an external opposite potential is applied on the electrochemical cell, the reaction continues to take place till the opposite voltage reaches the value 1.1 V. At this stage, no current flow through the cell and if there is any further increase in the external potentia1(EeJ, then reaction starts functioning in opposite direction i.e. an electrochemical cell behaves like an electrolytic cell.
Eexternal < Ecell
MCQ Electrochemistry Class 12 Chapter 3 Question 10.
ELectrode potential for Mg electrode varies according to the equation:
\(\mathrm{E}_{\mathrm{Mg}^{2+} \mathrm{Mg}}=\mathrm{E}_{\mathrm{Mg}^{2+} \mathrm{Mg}}^{\circ}-\frac{0.059}{2} \log \frac{1}{\left[\mathrm{Mg}^{2+}\right]}\)
The graph of \(\mathrm{E}_{\mathrm{Mg}^{2+} / \mathrm{Mg}}\) , vs. log [Mg2+] is
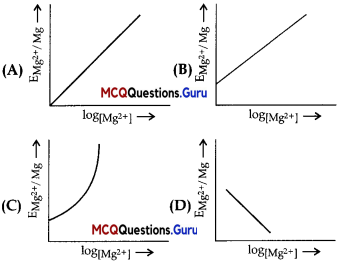
Answer:
Option (B) is correct.
Explanation:
\(\mathrm{E}_{\mathrm{Mg}^{2+} \mathrm{Mg}}=\mathrm{E}_{\mathrm{Mg}^{2+} \mathrm{Mg}}^{\circ}-\frac{0.059}{2} \log \frac{1}{\left[\mathrm{Mg}^{2+}\right]}\)
Compare this equation with the equation of straight line y = mx + c.
The graph of \(\mathrm{E}_{\mathrm{Mg}^{2+} / \mathrm{Mg}}\) vs. log [Mg2+] is a straight line with a positive slope and intercept \(\mathrm{E}_{\mathrm{Mg}^{2+} / \mathrm{Mg}}\)
MCQ Of Electrochemistry Chemistry Chapter 3 Question 11.
In an electrochemical process, a salt bridge is used
(A) as a reducing agent
(B) as an oxidizing agent
(C) to complete the circuit so that current can flow
(D) None of these
Answer:
(C) to complete the circuit so that current can flow
Explanation:
In an electrochemical cell, a salt bridge is used to complete the circuit so that current can flow.
![]()
Class 12 Electrochemistry MCQ Chemistry Question 12.
Following reactions occur at cathode during the electrolysis of aqueous silver chloride solution:
\(\mathrm{Ag}^{+}(\mathrm{aq})+\mathrm{e}^{-} \rightarrow \mathrm{Ag}(\mathrm{s})\) E0 = +0.80 V
\(\mathrm{H}^{+}(\mathrm{aq})+\mathrm{e}^{-} \rightarrow \frac{1}{2} \mathrm{H}_{2}(\mathrm{~g})\) E0 = +0.80 V
On the basis of their standard reduction electrode potential (E°) vaLues, which reaction is feasible at the cathode?
(A) Ag+(aq) + e– → Ag(s) E0 = + 0.80 V
(B) H+(aq) + e– → \(\frac {1}{2}\) H2(g) E0 = 0.00 V
(C) Both reactions are feasible
(D) None of the above
Answer:
(A) Ag+(aq) + e– → Ag(s) E0 = + 0.80 V
Explanation:
Ag+(aq) + e– → Ag(s) E0 = + 0.80 V
H+(aq) + e– → \(\frac {1}{2}\) H2(g) E0 = 0.00 V
On the basis of their standard reduction potential (E0) values, cathode reaction is given by the one with higher E0 values. Thus, Ag+(aq) + e– → Ag(s) reaction will be more feasible at cathode.
MCQ Questions For Class 12 Chemistry Chapter 3 Question 13.
Consider the following reaction: Cu(s) + 2Ag+(aq) → 2Ag(s) + Cu2+(aq) Depict the galvanic cell in which the given reaction takes place.
(A) Cu2+ (aq)|Cu(s) ||Ag+(aq)|Ag(s)
(B) Cu(s) | Cu2+(aq) || Ag+ (aq)|Ag(s)
(C) Ag+(aq)|Ag(s)||Cu2+(aq)|Cu(s)
(D)Ag+(s)|Ag+(aq)||Cu2+(aq)|Cu(s)
Answer:
(B) Cu(s) | Cu2+(aq) || Ag+ (aq)|Ag(s)
Explanation:
Oxidation half reaction
Cu(s) + Cu2+(aq) + 2e– .
Reduction half reaction

Chapter 3 Chemistry Class 12 MCQ Question 14.
Which of the following statements is not correct?
(A) Copper liberates hydrogen from acids.
(B) In its higher oxidation states, manganese forms stable compounds with oxygen and fluorine.
(C) Mn3+ and CO3+ are oxidising agents in aqueous solution.
(D) Ti3+ and Cr2+ are reducing agents in aqueous solution.
Answer:
(A) Copper liberates hydrogen from acids.
Explanation:
Copper does not liberate hydrogen from adds because copper lies below hydrogen in electrochemical series. So, copper does not have sufficient electrode potential to liberate elemental hydrogen form compounds in which oxidation state of hydrogen is +1.
MCQ On Electrochemistry Class 12 Pdf Question 15.
Calculate the emf of the following cell at 298 K: Mg(s)|Mg2 (0.1 M)||Cu2+ (1.0 x 10-3M)|Cu(s) [Given E0cell = 2.71 V]
(A) 1.426 V
(B) 2.503 V
(C) 2.651 V
(D) 1.8 V
Answer:
(C) 2.651 V
Explanation:
Ecell = \(E_{\text {Cell }}^{0}-\frac{0.059}{n} \log \frac{\left[\mathrm{Mg}^{2+}\right]}{\left[\mathrm{Cu}^{2+}\right]}\)
= \(2.71 \mathrm{~V}-\frac{0.059}{2} \log \frac{0.1}{0.001}\)
= \(2.71 \mathrm{~V}-\frac{0.059}{2} \log 10^{2}\)
Ecell = 2.651V
![]()
MCQs On Electrochemistry Chemistry Chapter 3 Question 16.
Reduction potentials of some ions are given below. Arrange them in decreasing order of oxidising power.
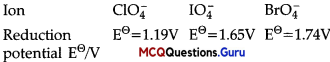
(A) ClO–4> IO–4> BrO–4
(B) IO–4> BrO–4> ClO–4
(C) BrO–4> IO–4> ClO–4
(D) BrO–4> ClO–4> IO–4
Answer:
(C) BrO–4> IO–4> ClO–4
Explanation:
Higher the reduction pøtential, higher is its tendency to get reduced. Hence, the order of oxidising power is: ClO–4> IO–4> BrO–4
Electrochemistry Class 12 MCQ Questions Question 17.
Using the data given below find strongest reduction agent.
\(\mathrm{E}_{\mathrm{Cr}_{2} \mathrm{O}_{7}^{2}-\mathrm{Cr}^{3+}}^{-1}=1.33 \mathrm{~V}, \mathrm{E}_{\mathrm{Cl}_{2} / \mathrm{Cl}^{-}}\) = 1.36 V
\(\mathrm{E}_{\mathrm{MnO}_{4} / \mathrm{Mn}^{2+}}^{-}=1.51 \mathrm{~V}, \mathrm{E}_{\mathrm{Cr}^{3} / \mathrm{Cr}}^{-}\) = – 0.74 V
(A) Cl–
(B) Cr
(C) Cr3+
(D) Mn2+
Answer:
(B) Cr
Explanation:
The negative value of standard reduction potential for Cr to Cr means that the redox couple is a stronger reducing agent.
Chemistry Class 12 Chapter 3 MCQ Question 18.
What will happen during the electrolysis of aqueous solution of CuSO4 by using platinum electrodes?
(A) Copper will deposit at cathode.
(B) Copper will deposit at anode.
(C) Oxygen will be released at anode.
(D) Copper will dissolve at anode.
Answer:
(C) Oxygen will be released at anode.
Explanation:
CuSO4 ⇌ Cu2+ + SO2-4
H2O = H+ + OH–
At cathode, .
Cu2+ + 2e2- → Cu; EΘcell = 0.34 V
H+ + e– → \(\frac {1}{2}\); H2EΘcell= 0.00 V
This reaction will take place due to higher reduction potential.
At anode,
2SO2- + 2e– → S2O2- + 2e8 EΘcell = 1. 96 V
2H2O → O2 + 4H+ + 4e– EΘcell = 1.23 V
The reaction with lower value of E0 will be preferred a t anode, hence O2 is released at anode.
![]()
Class 12 Chemistry Ch 3 MCQ Question 19.
What will happen during the electrolysis of aqueous solution of CuSO4 in the presence of Cu electrodes?
(A) Copper will deposit at cathode.
(B) Copper will dissolve at anode.
(C) Oxygen will be released at anode.
(D) Copper will deposit at anode.
Answer:
(A) Copper will deposit at cathode.
Explanation:
Electrolysis of CuSO4 can be represented by two half-cell reactions these occurring at cathode and anode, respectively, as given below:
At cathode: Cu2+ + 2e2- → Cu(s)
At anode : Cu(s) →Cu2+ + 2e–
In above reaction Cu will deposit at cathode while copper will dissolve at anode. Hence, (a) and (b) are the correct options.
Ch 3 Chemistry Class 12 MCQ Question 20.
Conductivity K, is equal to …………..
(A) \(\wedge_{m}\)
(B) \(\frac {G}{R}\)
(C) \(\frac {1}{A}\)
(D) All of the above
Answer:
(B) \(\frac {G}{R}\)
Explanation:
k = \(\frac{1}{R} \cdot \frac{1}{A} \text { or } \frac{G}{R}\)
Assertion And Reason Based MCQs (1 Mark each)
Directions: In the following questions, A statement of Assertion (A) is followed by a statement of Reason (R). Mark the correct choice as.
(A) Both A and R are true and R is the correct explanation of A
(B) Both A and R are true but R is NOT the correct explanation of A
(C) A is true but R is false
(D) A is false and R is True
Question 1.
Assertion (A): Conductivity of an electrolyte increases with decrease in concentration.
Reason (R): Number of ions per unit volume decreases on dilution.
Answer:
(D) A is false and R is True
Explanation:
Conductivity of an electrolyte I decreases with decrease in concentration because of ions per unit volume decreases on dilution.
Question 2.
Assertion (A): \(\Lambda_{\mathrm{m}}\) for weak electrolytes shows a sharp increase when the electrolytic solution is diluted. The reaction with lower value of E0 will be preferred at anode, hence 02 is released at anode.
Reason (R): For weak electrolytes degree of dissociation increases with dilution of solution.
Answer:
(A) Both A and R are true and R is the correct explanation of A
Explanation:
Weak electrolytes dissociate partially in concentrated solution. On dilution, their degree of dissociation increases hence, their A. increases sharply.
![]()
Question 3.
Assertion (A): Electrolytic conduction increases with increase in temperature.
Reason (R): Increase in temperature cause the electronic movement more rapid
Answer:
(C) A is true but R is false
Explanation:
As the temperature of electrolytic solution is increased, the kinetic energy of the ion increases. This results in the increase of electrical conductance of electrolytic solutions.
Question 4.
Assertion (A): Molar Conductivity of an ionic solution depends on temperature.
Reason (R): Molar Conductivity of an ionic solution depends on the concentration of electrolytes in the solution.
Answer:
(B) Both A and R are true but R is NOT the correct explanation of A
Explanation:
Molar Conductivity of .an ionic J solution depends on (he temperature as well E as on the i omentiation of the electrolytes ini Ihe solution.
Question 5.
Assertion (A): EceU should have a positive value for the cell to function.
Reason (R): Ecathode < Eanode
Answer:
(C) A is true but R is false
Explanation:
Ecell = Ecathode – Eanode To have positive value of Ecell, ECathode should be
greater than Eanode
Question 6.
Assertion (A): Cu is less reactive than hydrogen.
Reason (R): \(\mathrm{E}_{\mathrm{Cu}^{2+} / \mathrm{Cu}}^{\Theta}\) is negative.
Answer:
(C) A is true but R is false.
Explanation:
Cu is less reactive than hydrogen because \(\mathrm{E}_{\mathrm{Cu}^{2+} / \mathrm{Cu}}^{\Theta}\) is positive.
![]()
Question 7.
Assertion (A): Copper sulphate can be stored in zinc vessel.
Reason (R): Zinc is more reactive than copper.
Answer:
(D) A is false and R is True
Explanation:
Zinc will get dissolved in CuSO4 solution, since, zinc is more reactive than copper.
Question 8.
Assertion (A): \(\mathrm{E}_{\mathrm{Ag}^{+} / \mathrm{Ag}}\) increases with increase in concentration of Ag+ ions.
Reason (R): \(\mathrm{E}_{\mathrm{Ag}^{+} / \mathrm{Ag}}\) has a positive value.
Answer:
(B) Both A and R are true but R is NOT the correct explanation of A
Explanation:
Ag + e– → Ag
\(\mathrm{E}_{\mathrm{Ag}^{+} / \mathrm{Ag}}=\mathrm{E}^{\Theta}{\mathrm{Ag}^{\prime} / \mathrm{Ag}}-\frac{\mathrm{RT}}{\mathrm{nF}} \log \frac{1}{\left[\mathrm{Ag}^{+}\right]}\)
On increasing [Ag4], EAg+/ Ag will increase and it will increase and it has a positive value.
Question 9.
Assertion (A): Electrolysis of NaCl solution gives chlorine at anode instead of O2.
Reason (R): Formation of oxygen at anode requires over voltage.
Answer:
(A) Both A and R are true and R is the correct explanation of A
Explanation:
Formation of oxygen has lower I value of I:° than formation of chlorine even then it is not formed because it requires over voltage.
Case-Based MCQS’
I. Read the passage given below and answer the following questions:
The cell constant is usually determined by measuring the resistance of the cell containing a solution whose conductivity is already known. For this purpose, we generally use KCl solutions whose conductivity is known accurately at various concentrations and at different temperatures. Consider the resistance of a conductivity cell filled with 0.1 M KCl solution is 200 Ohm. If the resistance of the same cell when filled with 0.02 M KCl solution is 420 Ohm. (Conductivity of 0.1 M KCl solution is 1.29 Sm-1.) The following questions are Multiple Choice
Questions. Choose the most appropriate answer:
Question 1.
What is the conductivity of 0.02 M KCl solution?
(A) 0.452 S m-1
(B) 0.215 S m-1
(C) 0.614 S m-1
(D) 0.433 S m-1
Answer:
(C) 0.614 S m-1
Explanation:
Conductivity of 0.02 mol L-1 KCl Solution = Cell constant resistance \(\frac {258}{420}\) = 0.614 Sm-1
![]()
Question 2.
What will happen to the conductivity of the cell with the dilution ?
(A) First decreases then increases
(B) Increases
(C) First increases then decreases
(D) Decreases
Answer:
(D) Decreases
Explanation:
The conductivity decreases with dilution.
Question 3.
The cell constant of a conductivity cell …………
(A) Changes with change of electrolyte.
(B) Changes with change of concentration of electrolyte.
(C) Changes with temperature of electrolyte.
(D) Remains constant for a cell.
Answer:
(D) Remains constant for a cell.
Explanation:
The cell constant of a conductivity cell remains constant for a cell.
Question 4.
SI unit for conductivity of a solution is ………….
(A) S m-1
(B) Sm2 mol-1
(C) mol cm-3
(D) S cm2 mol-1
Answer:
(A) S m-1
Explanation:
SI unit for conductivity of a solution is Sm-1
OR
Which of the following is not true? The conductivity of solutions of different electrolytes in the same solvent and at a given temperature differs due to
(A) size of the ions in which they dissociate
(B) concentration of ions
(C) charge of the ions in which they dissociate
(D) is independent of ions movement under a potential gradient
Answer:
(D) is independent of ions movement under a potential gradient
Explanation:
The conductivity of solutions of different electrolytes in the same solvent and at a given temperature differs due to size and charge of the ions in which they dissociate, concentration of ions, ease with which the ions move under a potential gradient.
II. Read the passage given below and answer the following questions:
A galvanic cell consists of a metallic zinc plate immersed in 0.1 M Zn(NO3)2 solution and metallic plate of lead in 0.02 M Pb(NO3)2 solution. The following questions are multiple choice questions. Choose the most appropriate answer:
![]()
Question 5.
How will the cell be represented ?
(A) Zn(s) | Zn2+(aq)| | Pb2+(aq)|Pb(s)
(B) Zn2+(s)| Zn(aq) | | Pb2+(aq)|Pb(s)
(C) Pb2+(aq)|Pb(s)| |Zn2+(s)| Zn(aq)
(D) Pb(s)|Pb2+(aq)| |Zn2+(s)| Zn(aq)
Answer:
(A) Zn(s) | Zn2+(aq)| | Pb2+(aq)|Pb(s)
Explanation:
Cell representation:
Zn(s) | Zn2+ (aq) | | Pb2+ (aq),|Pb(s)
Question 6.
Calculate the emf of the cell.
(A) 6.01 V
(B) 0.412 V
(C) 0.609 V
(D) 4.12 V
Answer:
(C) 0.609 V
Explanation:
According to Xernst equation:
\(\mathrm{E}_{\text {cell }}=\mathrm{E}_{\text {cell }}-\frac{0.0591}{2} \log \frac{\left[\mathrm{Zn}^{2+}\right]}{\left[\mathrm{Pb}^{2+}\right]}\)
\(\mathrm{E}_{\text {cell }}=[-0.13-(-0.76)]-\frac{0.0591}{2} \log \frac{0.1}{0.02}\)
= 0.63 – 0.02955 x log 5
= 0.63 – 0.02955 x 0.6990
= 0.63 – 0.0206 – 0.6094 V
Commonly Made Errors :
- The cell representation is given incorrectly by manv candidates.
- The calculation of emf of the cell by using Xernst equation is incorrect, in some cases.
Answering Tip :
- Do more practice of cell representation and numerical based on Nernst equation.
Question 7.
What product is obtained at cathode?
(A) Zn
(B) Pb
(C) Zn2+
(D) Pb2+
Answer:
(B) Pb
Explanation:
Anode reaction: Zn(s) → Zn2+ (aq) + 2e–
Cathode reaction: Pb2+(aq) + 2e – → Pb(s)
![]()
Question 8.
Which of the following statement is not correct about an inert electrode in a cell ?
(a) It does not participate in the cell reaction.
(b) It provides surface either for oxidation or for reduction reaction.
(c) It provides surface for conduction of electrons.
(d) It provides surface for redox reaction.
Answer:
(a) It does not participate in the cell reaction.
Explanation:
Inert electrode dot’s not participate in redox reaction and acts only as source or sink tor electrons. It provides surface either fori oxidation or for reduction reaction.
III. Products of electrolysis depend on the nature of material being electrolysed and the type of electrodes being used. If the electrode is inert (e.g., platinum or gold), it does not participate in the chemical reaction and acts only as source or sink for electrons. On the other hand, if the electrode is reactive, it participates in the electrode reaction. Thus, the products of electrolysis may be different for reactive and inert electrodes. Aqueous copper sulphate solution and aqueous silver nitrate solution are electrolysed by ampere current for 10 minutes in separate electrolytic cells. In these questions, a statement of assertion followed by a statement of reason. Choose the correct answer out of the following choices.
(A) Assertion and reason both are correct statements and reason is correct explanation for assertion.
(B) Assertion and reason both are correct statements but reason is not correct explanation for assertion.
(C) Assertion is correct statement but reason is wrong statement.
(D) Assertion is wrong statement but reason is correct statement.
Question 1.
Assertion (A): The mass of copper and silver, deposited on the cathode be same.
Reason (R): Copper and silver have different equivalent masses.
Answer:
(D) Assertion is wrong statement but reason is correct statement.
Explanation:
W = itE/96300 = 1 x 10 x 60 x 31.8/96500 for copper. It will be different for silver since the equivalent weight of silver is different.
Question 2.
Assertion (A): At equilibrium condition Ecell = 0 or ∆rG = 0.
Reason (R): Ecell is zero when both electrodes of the cell are of the same metal.
Answer:
(B) Assertion and reason both are correct statements but reason is not correct explanation for assertion.
Explanation:
At equilibrium, condition of Ecell = 0, ∆rG = 0
Question 3.
Assertion (A): The negative sign in the expression \(\mathrm{E}_{\mathrm{Zn}^{2}+/ \mathrm{Zn}}\) = – 0.76V means Zn2+cannot be oxidised to Zn.
Reason (R): Zn is more reactive than hydrogen & Zn will oxidised, & H+ will get reduced.
Answer:
(A) Assertion and reason both are correct statements and reason is correct explanation for assertion.
Explanation:
It shows that the reduced form of (Zn2+) is not stable. It is difficult to reduce Zn2+ to Zn. Rather the reverse reaction i.e Zn can get oxidised to Zn2+ and H~ will get reduced as it is stabler among both the reduced species.
![]()
Question 4
Assertion (A): In a galvanic cell, chemical energy is converted into electrical energy.
Reason (R): Redox reactions provide the chemical energy to the cell.
Answer:
(A) Assertion and reason both are correct statements and reason is correct explanation for assertion.
Explanation:
The redox reactions provide the chemical energy’ to the galvanic cell which is converted into electrical energy.
OR
Assertion (A): Copper sulphate cannot be stored in zinc vessel.
Reason (R): Zinc is less reactive than copper.
Answer:
(C) Assertion is correct statement but reason is wrong statement.
Explanation:
Copper sulphate cannot be stored in zinc vessel as zinc is more reactive than copper.