Solutions Class 12 MCQs Questions with Answers
Solutions Class 12 MCQ Chapter 2 Question 1.
A molar solution is one that contains one mole of a solute in
(A) 1000 g of the solvent
(B) one litre of the solvent
(C) one litre of the solution
(D) 22.4 litre of the solution
Answer:
(C) one litre of the solution
Explanation:
A molar solution is one that contains one mole of a solute in one litre of the solution.
![]()
Solution MCQ Class 12 Chapter 2 Question 2.
In which mode of expression, the concentration of a solution remains independent of temperature?
(A) Molarity
(B) Normality
(C) Formality
(D) Molality
Answer:
(D) Molality
Explanation:
The molality of a solution does not change with temperature.
![]()
Solution MCQ Chapter 2 Question 3.
The increase in the temperature of the aqueous solution will result in its
(A) Molarity to increase
(B) Molarity to decrease
(C) Mole fraction to increase
(D) Mass % to increase
Answer:
(B) Molarity to decrease
Explanation:
An increase in temperalure increase llie volume of solution and therefore will result in its molarity to decrease.
Class 12 Chemistry Chapter 2 MCQ Question 4.
KH value for Ar(g), CO2(g), HCHO(g) and CH4(g) are 4.039, 1.67, 1.83 x 10.5, and 0.143, respectively. Arrange these gases
in the order of their increasing solubility
(A) HCHO < CH4 < CO2< Ar
(B) HCHO < CO2 < CH4 < Ar
(C) Ar < CO2 < CH4 < HCHO
(D) Ar < CH4 < C02 < HCHO
Answer:
(C) Ar < CO2 < CH4 < HCHO
Explanation:
According to Henry’s law,
P = KHC
KH ∝ \(\frac {1}{C}\)
Where P = Partial pressure of gas C = Concentration of gas
KH = Henry’s constant
It implies that as the value of KH increases, mole fraction of gas solute in solvent decreases. Hence, higher the KH value, lower is the solubility of gas.
The order of increasing solubility of gases in
Ar < CO2 < CH4 < HCHO
MCQ Questions For Class 12 Chemistry Chapter 2 Question 5.
A beaker contains a solution of substance ‘A’. Precipitation of substance ‘A’ takes place when small amount of ‘A’ is added to the solution. The solution is …………
(A) saturated
(B) supersaturated
(C) unsaturated
(D) concentrated
Answer:
(B) supersaturated
Explanation:
When a small amount of solute is added to its solution, it does not dissolve and get precipitated then this type of solution is called as supersaturated solution.
![]()
Solution Class 12 MCQ Chapter 2 Question 6.
At equilibrium the rate of dissolution of a solid solute in a volatile liquid solvent is …………..
(A) less than the rate of crystallisation
(B) greater than the rate of crystallisation
(C) equal to the rate of crystallisation
(D) zero
Answer:
(C) equal to the rate of crystallisation
Explanation:
In an equilibrium state, the rate of dissolution of a solid solute in a volatile liquid solvent is equal to the rate of crystallization.
MCQ On Solutions Class 12 Chapter 2 Question 7.
Which of the following aqueous solutions should have the highest boiling point?
(A) 1.0 M NaOH
(B) 1.0 M Na2S02
(C) 1.0 M NH4NO3
(D) 1.0 M KN03
Answer:
(B) 1.0 M Na2S02
Explanation:
1.0 M Na2S04 since it furnishes I maximum number of ions (2Na+ + S024 ‘).
Class 12 Solutions MCQ Chapter 2 Question 8.
When 1 mole of benzene is mixed with 1 mole of toluene the vapour will contain : (Given: vapour of benzene = 12.8kPa and vapour pressure of toluene = 3.85 kPa).
(A) equal amount of benzene and toluene as it forms an ideal solution
(B) unequal amount of benzene and toluene as it forms a non ideal solution
(C) higher percentage of benzene
(D) higher percentage of toluene
Answer:
(C) higher percentage of benzene
Explanation:
When 1 mole of benzene is mixed with 1 mole of toluene the vapour will contain higher percentage of benzene. As it is an ideal solution, it follows Raoult’s law. The vapour pressure of the solution depends on the mole fraction of the solvent. PSoln = XSolventP0Solvent PSoln is the vapour pressure of the solution X is the mole fraction of the solvent P0Solvent is the vapour pressure of the pure solvent Since the mole fraction of both the components is same, but the vapour pressure of benzene is higher than the toluene, its percentage will be greater in the vapour of the solution.
![]()
MCQ Solutions Class 12 Chapter 2 Question 9.
If two liquids A and B form minimum boiling azeotrope at some specific composition then …………..
(A) A-B interactions are stronger than those between A-A or B-B.
(B) Vapour pressure of solution increases because more number of molecules of liquids A and B can escape from the solution.
(C) Vapour pressure of solution decreases because less number of molecules of only one of the liquids escape from the solution.
(D) A-B interactions are weaker than those between A-A or B-B.
Answer:
(D) A-B interactions are weaker than those between A-A or B-B.
Explanation:
When solute-solvent or A-B interactions are weaker than the A-A or B-B interactions, molecules of A or B will find it easier to escape than in pure state. This will increase the vapour pressure and result in positive deviation from Raoult’s law. Such solutions are called minimum boiling azeotropes.
Solutions MCQ Class 12 Chapter 2 Question 10.
For a dilute solution, Raoult’s law states that
(A) The lowering of vapour pressure is equal to the mole fraction of solute.
(B) The relative lowering of vapour pressure is equal to the mole fraction of solute.
(C) The relative lowering of vapour pressure is proportional to the amount of solute in solution.
(D) The vapour pressure of the solution is equal to the mole fraction of the solute.
Answer:
(B) The relative lowering of vapour pressure is equal to the mole fraction of solute.
Explanation:
According to Raoult’s law, for a dilute solution, the relative lowering of vapour pressure is equal to the mole fraction of solute.
\(\frac{P_{A}^{0}-P_{A}}{P^{0}}\) = XB
Where
\(\frac{P_{A}^{0}-P_{A}}{P^{0}}\) = Relative lowering of vapour pressure
XB = mole fraction of solute
Chapter 2 Chemistry Class 12 MCQ Question 11.
Which of the following units is useful in relating concentration of solution with its vapour pressure?
(A) Mole fraction
(B) Parts per million
(C) Mass percentage
(D) Molality
Answer:
(A) Mole fraction
Explanation:
Mole fraction is used in relating vapour pressure with concentration of solution and according to the Raoult’s law, the partial vapour pressure of each component in the solution is directly proportional to its mole fraction.
![]()
Chemistry Class 12 Chapter 2 MCQ Question 12.
The unit of ebullioscopic constant is :
(A) K kg mol-1 or K (molality)-1
(B) mol kg-1 K-1 or K-1 (molality)
(C) kg mol-1 K-1 or K’-1 (molality)’
(D) K mol kg-1 or K (molality)
Answer:
(A) K kg mol-1 or K (molality)-1
Explanation:
It is the unit of ebullioscopic constant (K).
k = K kg mol-1 or K (molality)-1
Solutions Chemistry Class 12 MCQ Chapter 2 Question 13.
Value of Henry’s constant KH is …………..
(A) Increases with increase in temperature.
(B) Decreases with increase in temperature
(C) Remains constant
(D) First increases then decreases.
Answer:
(A) Increases with increase in temperature.
Explanation:
Value of Henry’s constant increases with increase in temperature.
![]()
Solution Class 12 Chemistry MCQ Chapter 2 Question 14.
Considering the formation, breaking and strength of hydrogen bond, predict which of the following mixtures will show a positive deviation from Raoul t’s law?
(A) Methanol and acetone.
(B) Chloroform and acetone.
(C) Nitric acid and water
(D) Phenol and aniline.
Answer:
(A) Methanol and acetone.
Explanation:
Mixture of methanol and acetone exhibits positive deviation because methanol-1 i methanol and acetone-acetone interaction is more than methanol-acetone. he more number of hydrogen bonds are broken the less number of new hydrogen bonds are formed.
Class 12 Chemistry Solutions MCQ Questions Question 15.
If two liquids A and B form minimum boiling azeotrope at some specific composition, then.
(A) A-B interactions are stronger than those between A-A or B-B.
(B) vapour pressure of solution increases because more number of molecules of liquids A and B can escape from the solution.
(C) vapour pressure of solution decreases because less number of molecules of only one of the liquids escape from the solution.
(D) A-B interactions are weaker than those between A-A or B-B.
Answer:
(A) A-B interactions are stronger than those between A-A or B-B.
Explanation:
It A-B interactions is less than A-A or B-B The vapour pressure will be more and the result will be positive deviation. The solutions which exhibits positive deviation form minimum boiling azeotropes.
![]()
Ch 2 Chemistry Class 12 MCQ Question 16.
Consider the figure and mark the correct option.
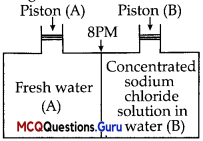
(A) Water will move from side (A) to side (B) if pressure lower than osmotic pressure is applied on piston (B).
(B) Water will move from side (B) to side (A) if pressure greater than osmotic pressure is applied on piston (B).
(C) Water will move from side (B) to side (A) if pressure equal to osmotic pressure is applied on piston (B).
(D) Water will move from side (A) to side (B) if pressure equal to osmotic pressure is applied on piston (A).
Answer:
(B) Water will move from side (B) to side (A) if pressure greater than osmotic pressure is applied on piston (B).
Explanation:
Water will move from side (B) to side (A) if a pressure greater than osmotic pressure is applied on piston (B). This is a process of reverse osmosis.
Solution Chapter 2 MCQ Chapter 2 Question 17.
Which of the following solutions in water has highest boiling point?
(A) 1 M NaCl
(B) 1 M MgCl2
(C) 1 M urea
(C) 1 M glucose
Answer:
(B) 1 M MgCl2
Explanation:
MgCl2 in aqueous solution gives maximum number of ions than other solutions. So, it has highest boiling point.
![]()
Solutions Class 12 Chemistry MCQ Question 18.
Relative lowering of vapour pressure is a colligative property because ………….
(A) It depends on number of particles of electrolyte solute in solution and does not depend on the nature of the solute particles.
(B) It depends on the concentration of a non-electrolyte solute in solution as well as on the nature of the solute molecules.
(C) Is depends on the concentration of an electrolyte or non-electrolyte solute is solution as well on the nature of solute molecules.
(D) None of the above
Answer:
(A) It depends on number of particles of electrolyte solute in solution and does not depend on the nature of the solute particles.
Explanation:
Colligative property depends on the number of solute particles and not on the nature of the particles.
Assertion And Reason Based MCQs (1 Mark each)
Directions: In the following questions, A statement of Assertion (A) is followed by a statement of Reason (R). Mark the correct choice as.
(A) Both A and R are true and R is the correct explanation of A
(B) Both A and R are true but R is NOT the correct explanation of A
(C) A is true but R is false
(D) A is false and R is True
Class 12 Chemistry Solutions MCQ Question 1.
Assertion (A): A molar solution is more
concentrated than molal solution.
Reason (R): A molar solution contains one mole of solute in 1000 mL of solution.
Answer:
(A) Both A and R are true and R is the correct explanation of A
Explanation:
A molar solution is more concentrated than molal solution because molar solution contains 1 mole of solute in 1 litre of the solution which include both solute and solvent.
MCQ Of Solutions Class 12 Chapter 2 Question 2.
Assertion (A): Molarity of 0.1 N solution of HCl is 0.1 M.
Reason (R): Normality and molarity of a solution are always equal.
Answer:
(C) A is true but R is false
Explanation:
Normality and molarity of a solution are not alwavs equal. Normality depends on chemical equivalent of the substance while molaritv depends on molecular mass of the substance.
![]()
MCQ On Solution Class 12 Chapter 2 Question 3.
Assertion (A): Molarity of a solution changes with temperature.
Reason (R): Molarity is dependent on volume of solution.
Answer:
(A) Both A and R are true and R is the correct explanation of A
Explanation:
As molarity is dependent on volume of solution and volume rises with increase in temperature. Molaritv is inversely proportional to temperature. So, ns temperature increases, volume increases and molarity decreases.
Class 12 Chemistry Ch 2 MCQ Question 4.
Assertion (A): Molarity of a solution in liquid state changes with temperature.
Reason (R): The volume of a solution changes with change in temperature.
Answer:
(A) Both A and R are true and R is the correct explanation of A
Explanation:
Molarity changes with temperature I because volume changes with temperature.
Question 5
Assertion (A): An ideal solution obeys Henry’s law.
Reason (R): In an ideal solution, solute-solute as well as solvent-solvent interactions are similar to solute- solvent interaction. [CBSE Delhi Set-Ill,
Answer:
(D) A is false and R is True
Explanation:
An ideal solution obeys Raoult’s law.
![]()
Question 6.
Assertion (A): Dimethyl ether is less volatile than ethyl alcohol.
Reason (R): Dimethyl ether has greater vapour pressure than ethyl alcohol.
Answer:
(D) A is false and R is True
Explanation:
Dimethyl ether is more volatile I than ethyl alcohol.
Question 7.
Assertion (A): Vapour pressure increase with increase in temperature.
Reason (R): With increase in temperature, more molecules of the liquid can go into vapour phase.
Answer:
(A) Both A and R are true and R is the correct explanation of A
Explanation:
Vapour pressure increase with increase in temperature because more molecules of the liquid can go into vapour phase with increase in temperature.
Question 8.
Assertion (A): Elevation in boiling point is a colligative property.
Reason (R): Elevation in boiling point is directly proportional to molarity.
Answer:
(A) Both A and R are true and R is the correct explanation of A
Explanation:
Elevation in boiling point is a colligative property. It is directly proportional to molarity.
Question 9.
Assertion (A): 0.1 M solution of KC1 has great osmotic pressure than 0.1 M solution of glucose at same temperature.
Reason'(R): In solution KC1 dissociates to produce more number of particles.
Answer:
(A) Both A and R are true and R is the correct explanation of A
Explanation:
KCl is ionic compound, hence dissociates into ions but glucose is a covalent = compound which does not dissociate into ions.
![]()
Case-Based MCQS’
I. Read the passage given below and answer the following questions:
Scuba apparatus includes a tank of compressed air toted by the diver on his or her back, a hose for carrying air to a mouthpiece, a face mask that covers the eyes and nose, regulators that control air flow, and gauges that indicate depth and how much air remains in the tank. A diver who stays down too long, swims too deep, or comes up too fast can end up with a condition called “the bends.” In this case, bubbles of gas in the blood can cause intense pain, even death.
In these following questions a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
(A) Assertion and Reason both are correct statements and Reason is correct explanation for Assertion.
(B) Assertion and Reason both are correct statements but Reason is not correct explanation for Assertion.
(C) Assertion is correct statement but Reason is wrong statement.
(D) Assertion is wrong statement but Reason is correct statement.
Question 1.
Assertion : Scuba divers may face a medical condition called ‘bends’.
Reason : ‘Bends’ can be explained with the help of Henry’s law as it links the partial pressure of gas to that of its mole fraction.
Answer:
(A) Assertion and Reason both are correct statements and Reason is correct explanation for Assertion.
Explanation:
Henry’s law explains some biological phenomena like the ‘bends’ experienced by the scuba divers. Since mole fraction of a gas in the solution is a measure of its solution)
Question 2.
Assertion : Bends is caused due to formation of nitrogen bubbles in the blood of scuba divers which blocks the capillaries.
Reason : Underwater high pressure increases solubility of gases in blood, while as pressure gradually decreases moving towards the surface, gases are released and nitrogen bubbles are formed in blood.
Answer:
(A) Assertion and Reason both are correct statements and Reason is correct explanation for Assertion.
Explanation:
Scuba divers must cope with high concentrations of dissolved gases while breathing air at high pressure underwater. Increased pressure increases the solubility of atmospheric gases in blood. When the divers come towards surface, the pressure gradually decreases. This releases the dissolved gases and leads to the formation of bubbles of nitrogen in the blood. This blocks capillaries and creates a medical condition known as bends.
![]()
Question 3.
Assertion: Soft drinks and soda water bottles are sealed under high pressure.
Reason : High pressure maintains the taste and texture of the soft drinks.
Answer:
(C) Assertion is correct statement but Reason is wrong statement.
Explanation:
The bottle is sealed under high pressure to increase the solubility of C02 in soft drinks and soda water.
Question 4.
Assertion : Anoxia is a condition experienced by climbers which makes them suddenly agile and unable to think clearly.
Reason : At high altitudes the partial pressure of oxygen is less than that at the ground level.
Answer:
(D) Assertion is wrong statement but Reason is correct statement.
Explanation:
At high altitudes the partial pressure of oxygen is less than that at the ground level. This leads to low concentrations of oxygen in the blood and tissues of people living at high altitudes or climbers. This leads to a condition 3 called anoxia caused due to low oxygen in blood, making the climbers to become weak and unable to think clearly.
OR
Assertion : Solubility of gases in liquids decreases with rise in temperature.
Reason : As dissolution is an exothermic process, the solubility should decrease with increase of temperature.
Answer:
(A) Assertion and Reason both are correct statements and Reason is correct explanation for Assertion.
Explanation:
Solubility of gases in liquids decreases with rise in temperature. As dissolution is an exothermic process, the solubility should ? decrease with increase of temperature.
II. Read the passage given below and answer the following questions:
Raoult’s law states that for a solution of volatile liquids, the partial vapour pressure of each component of the solution is directly proportional to its mole fraction present in solution. Dalton’s law of partial pressure states that the total pressure (ptota[) over the solution phase in the container will be the sum of the partial pressures of the components of the solution and is given as : Ptotal = P1 + P2
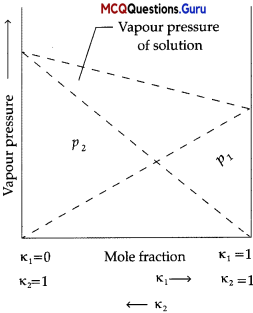
Question 1.
What type of deviation from Raoult’s law does the above graph represent ?
(A) First positive then negative
(B) Negative deviation
(C) Positive deviation
(D) First negative then positive
Answer:
(B) Negative deviation
Explanation:
Negative deviation
![]()
Question 2.
In comparison to a 0.01 M solution of glucose, the depression in freezing point of a 0.01 M MgCl2 solution is …………
(A) the same
(B) about twice
(C) about three times
(D) about six times
Answer:
(C) about three times
Explanation:
∆Tf = i kf m, where i = 1 for glucose.
∆Tglucosef = 1 x Kf x 0.01
In case of MgCl2 —> Mg2+ + 2Cl– where i = 3,
ø \(\mathrm{T}_{f}^{\mathrm{MgCl}_{2}}\) = 3 x 0.01 x Kf
ø \(\mathrm{T}_{f}^{\mathrm{MgCl}_{2}}\) = 3 x ∆Tglucosef
Hence, the depression in freezing point of MgCU is three times that of glucose.
Question 3.
A solution of two liquids boils at a temperature more than the boiling point of either of them. What type of deviation will be shown by the solution formed in terms of Raoult’s law ?
(A) Negative deviation
(B) Positive deviation
(C) First positive then negative
(D) First negative then positive
Answer:
(A) Negative deviation
Explanation:
Since the Boiling point of the solution is more than the Boiling point of the individual components in the solution, it indicates that the vapour pressure exerted by the solution is less than the expected, as boiling starts when vapour pressure equals the atmospheric pressure. Hence, the solution shows a negative deviation from the Raoult’s law,
Question 4.
Which of the following aqueous solutions should have the highest boiling point ?
(A) 1.0 M NaOH
(B) 1.0 M Na2S04
(C) 1.0MNH4NO3
(D) 1.OMKNO3
Answer:
(B) 1.0 M Na2S04
Explanation:
Na2SO4 will release 3 moles of ions/’ moles of Na2SO4 in the aqueous solution, and Boiling point being a colligative property, the Boiling point of this solution will be the highest as other solutions release only 2 ions each.
![]()
III. Read the passage given below and answer the following questions:
Boiling point or freezing point of liquid solution would be affected by the dissolved solids in the liquid phase. A soluble solid in solution has the effect of raising its boiling point and depressing its freezing point. The addition of non-volatile substances to a solvent decreases the vapour pressure and the added solute particles affect the formation of pure solvent crystals. According to many researches the decrease in freezing point directly correlated to the concentration of solutes dissolved in the solvent.
This phenomenon is expressed as freezing point depression and it is useful for several applications such as freeze concentration of liquid food and to find the molar mass of an unknown solute in the solution. Freeze concentration is a high quality liquid food concentration method where water is removed by forming ice crystals. This is done by cooling the liquid food below the freezing point of the solution.
The freezing point depression is referred as a colligative property and it is proportional to the molar concentration of the solution (m), along with vapour pressure lowering, boiling point elevation, and osmotic pressure. These are physical characteristics of solutions that depend only on the identity of the solvent and the concentration of the solute. The characters are not depending on the solute’s identity.
Question1.
When a non volatile solid is added to pure water it will:
(A) boil above 100°C and freeze above 0°C
(B) boil below 100°C and freeze above 0°C
(C) boil above 100°C and freeze below 0°C
(D) boil below 100°C and freeze below 0°C
Answer:
(B) boil below 100°C and freeze above 0°C
Question 2.
Colligative properties are:
(A) dependent only on the concentration of the solute and independent of the solvent’s and solute’s identity.
(B) dependent only on the identity of the solute and the concentration of the solute and independent of the solvent’s identity.
(C) dependent on the identity of the solvent and solute and thus on the concentration of the solute.
(D) dependent only on the identity of the solvent and the concentration of the solute and independent of the solute’s identity.
Answer:
(D) dependent only on the identity of the solvent and the concentration of the solute and independent of the solute’s identity.
Question 3.
Assume three samples of juices A, B and C have glucose as the only sugar present in them. The concentration of sample A, B and C are 0.1M, .5M and 0.2 M respectively. Freezing point will be highest for the fruit juice:
(a) A
(b) B
(c) C
(d) All have same freezing point
Answer:
(a) A
![]()
Question 4.
Identify which of the following is a colligative property:
(A) freezing point
(B) boiling point
(C) osmotic pressure
(D) all of the above
Answer:
(C) osmotic pressure