Chemical Kinetics Class 12 MCQs Questions with Answers
Chemical Kinetics Class 12 MCQ Chapter 4 Question 1.
The unit of rate constant depends upon the
(A) molecularity of the reaction
(B) activation energy of the reaction
(C) order of the reaction
(D) temperature of the reaction
Answer:
(C) order of the reaction
Explanation:
For the reaction A
xA + yB →Product
r = k[A]x[B]y
\(\frac {dx}{dt}\) = k[A]x[B]y
mol L-1s-1 = k(mol L-1 )(mol L-1 )y
\(k=\frac{\mathrm{mol} \mathrm{L}^{-1} \mathrm{~s}^{-1}}{\left(\mathrm{~mol} \mathrm{~L}^{-2}\right)^{x}\left(\mathrm{~mol} \mathrm{~L}^{-1}\right)^{y}}\)
(mol L-1)1-(x+y)S-1
Where (x + y) = order of the reaction
Chemical Kinetics MCQ Chapter 4 Question 2.
Compounds ‘X and ‘B’ react according to the following chemical equation:
A(g) + 2B(g) →2C(g) Concentration of either ‘X or ‘B’ were changed keeping the concentration of one of the reactants constant and rates were measured as function of initial concentration. Following results were obtained. Choose the Correct option for this reaction.
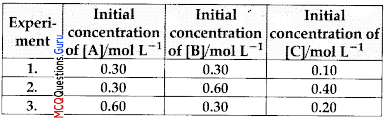
(A) Rate = k [A]2[B]
(B) Rate = k [A][B]
(C) Rate = k [A][B]2
(D) Rate k [A]2[B]0
Answer:
(B) Rate = k [A][B]
Explanation:
Suppose order with respect to A and B are x and y respectively.
Rate = k [A]x[B]y
For experiment 1,
0.1 = k(0.3)x(0.3)y ………(i)
For experiment 2,
0.4 = k (0.3)x(0.6)y ………(ii)
For experiment 3,
0.2 = k (0.6)x(0.3)y ………(iii)
Dividing equation (ii) by (i)
\(\frac{0.2}{0.1}=\frac{(0.6)^{y}}{(0.3)^{y}}\)
∴ y = 1
Dividing equation (iii) by (i)
\(\frac{0.2}{0.1}=\frac{(0.6)^{y}}{(0.3)^{y}}\)
∴ x = 1
Rate law
Rate = fc[A][B]2
![]()
MCQ On Chemical Kinetics Class 12 Question 3.
Consider the reaction A B. The concentration of both the reactants and the productsvaries expo nentially with time. Which of the foLlowing figures correctly describes the change in concentration of reactants and products with time?
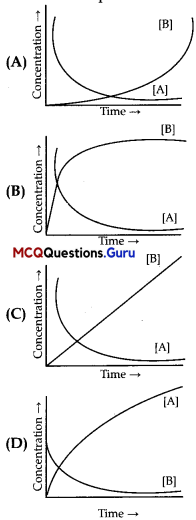
Answer:
Option (B) is correct.
Explanation:
As the reactant As concentration decreases with time, so the product B’s concentration increases. Also since the reaction is reversible, the increase and decrease in: concentration with respect to time is similar.
MCQ On Chemical Kinetics Chapter 4 Class 12 Question 4.
In the presence of a catalyst, heat evolved or absorbed during reaction:
(A) increases.
(B) decreases.
(C) remains unchanged.
(D) may increase or decrease.
Answer:
(C) remains unchanged.
Explanation:
Then is no effect on heat evolved or absorbed during the reaction in the presence t of a catalvst. It is because catalyst influence the rate of reaction and does not participate in the reaction.
Chemical Kinetics Class 12 MCQ Questions Question 5.
A graph of volume of hydrogen released vs. time for the reaction between zinc and dilute HC1 is given in figure. On the basis of this mark the correct option.
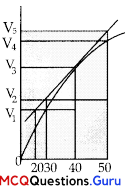
(A) Average rate up to 40 seconds is \(\frac{V_{3}-V_{2}}{40}\)
(B) Average rate up to 40 seconds is \(\frac{V_{3}-V_{2}}{40-30}\)
(C) Average rate up to 40 seconds is \(\frac{V_{3}}{40}\)
(D) Average rate up to 40 seconds is \(\frac{V_{3}-V_{2}}{40-20}\)
Answer:
(C) Average rate up to 40 seconds is \(\frac{V_{3}}{40}\)
Explanation:
Average rate of reaction up to 40 seconds on the basis of the graph is; \(\frac{V_{3}-0}{40-0}=\frac{V_{3}}{40}\)
![]()
Chemical Kinetics Class 12 MCQ Questions Question 6.
Which of the following statement is correct?
(A) The rate of a reaction decreases with passage of time as the concentration of reactants decreases.
(B) The rate of a reaction is same at any time during the reaction.
(C) The rate of a reaction is independent of temperature change.
(D) The rate of a reaction decreases with increase in concentration of reactant(s).
Answer:
(A) The rate of a reaction decreases with passage of time as the concentration of reactants decreases.
Explanation:
The rate of a reaction depends upon the concentration of reactants.
MCQ On Chemical Kinetics Class 12 Pdf Question 7.
Rate law cannot be determined from balancedchemical equation if:
(A) reverse reaction is involved.
(B) it is an elementary reaction.
(C) it is a sequence of elementary reactions.
(D) any of the reactants is in excess.
Answer:
Option (B) ¡s correct.
Explanation:
In case of elementary reaction the rate law can be determined fmm balanced chemical equation.
MCQ Questions For Class 12 Chemistry Chapter 4 Question 8.
Which of the following expressions is corred for the rate of reaction given below?
5Br– (aq) + BrO–3 (aq) + 6H+ (aq) → 3Br2 (aq)+ 3H2O (l)
(A) \(\frac{\Delta\left[\mathrm{Br}^{-}\right]}{\Delta t}=5 \frac{\Delta\left[\mathrm{H}^{+}\right]}{\Delta t}\)
(B) \(\frac{\Delta\left[\mathrm{Br}^{-}\right]}{\Delta t}=\frac{6}{5} \frac{\Delta\left[\mathrm{H}^{+}\right]}{\Delta t}\)
(C) \(\frac{\Delta\left[\mathrm{Br}^{-}\right]}{\Delta t}=\frac{5}{6} \frac{\Delta\left[\mathrm{H}^{+}\right]}{\Delta t}\)
(D) \(\frac{\Delta\left[\mathrm{Br}^{-}\right]}{\Delta t}=6 \frac{\Delta\left[\mathrm{H}^{+}\right]}{\Delta t}\)
Answer:
(C) \(\frac{\Delta\left[\mathrm{Br}^{-}\right]}{\Delta t}=\frac{5}{6} \frac{\Delta\left[\mathrm{H}^{+}\right]}{\Delta t}\)
Explanation:
Rate law expression for the above equation is written as:
5Br– (aq) + BrO–3 (aq) + 6H+ (aq) → 3Br2 (aq)+ 3H2O (l)
Rate law expression for the above equation can be presented as :
\(\frac{-1}{5} \frac{\Delta\left(\mathrm{Br}^{-}\right)}{\Delta t}=\frac{\Delta\left[\mathrm{BrO}_{3}\right]}{\Delta t}=\frac{-1}{6} \frac{\Delta\left[\mathrm{H}^{+}\right]}{\Delta t}=\frac{+1}{3} \frac{\Delta\left[\mathrm{Br}_{2}\right]}{\Delta t}\)
\(\frac{\Delta\left[\mathrm{Br}^{-}\right]}{\Delta t}=\frac{\Delta\left[\mathrm{Br} \mathrm{O}_{3}^{-}\right]}{\Delta t}=\frac{-5}{6} \frac{\Delta\left[\mathrm{H}^{+}\right]}{\Delta t}\)
\(\frac{\Delta\left[\mathrm{Br}^{-}\right]}{\Delta t}=\frac{5 \Delta\left[\mathrm{H}^{+}\right]}{6}\)
![]()
MCQ Of Chemical Kinetics Class 12 Question 9.
In a chemical reaction X → Y, it is found that the rate of reaction doubles when the concentration of X is increased four times. The order of the reaction with respect to X is
(A) 1
(B) 0
(C) 2
(D) 1/2
Answer:
(D) 1/2
Explanation:
X → Y
Rate(r) ∝ [X]n [Where n = Order of reaction]
If the concentration X is increased by 4 times X’ = 4X
Then, Rate(r’) ∝ [X’]n
\(\frac{r^{\prime}}{r}=\frac{[4 X]^{n}}{[X]^{n}}\) = 2
r’ is new rate, X’ is a new concentration
[4]n = 2
∴ n = \(\frac {1}{2}\)
Order of reaction = \(\frac {1}{2}\)
MCQ Chemical Kinetics Class 12 Ch 4 Question 10.
The half-life period for a zero order reaction is equal to
(A) \(\frac{0.693}{k}\)
(B) \(\frac{2 k}{[R]_{0}}\)
(C) \(\frac{2.303}{k}\)
(D) \(\frac{[R]_{0}}{2 k}\) (where [R]0 is initial concentration of reactant and k is rate constant).
Answer:
(D) \(\frac{[R]_{0}}{2 k}\)
Explanation:
Half life period of a zero order reaction = \(\frac{[R]_{0}}{2 k}\)
Where [R]0 = initial concentration of reactant k = Rate constant
MCQs Of Chemical Kinetics Class 12 Question 11.
For a zero order reaction, the slope in the plot of [R] vs. time is k
(A) \(\frac{[R]_{0}}{2 k}\)
(B) -K
(C) \(\frac{+k}{2.303}\)
(d) +K (where [R] is the final concentration of reactant)
Answer:
(B) -K
Explanation:
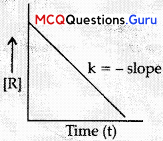
Chemical Kinetics MCQs Class 12 Chapter 4 Question 12.
A first order reaction is 50% completed in 1.26 x 1014 s. How much time would it take for 100% completion ?
(A 1.26 x 1015
(B) 2.52 x 1014s
(C) 2.52 x 1028s
(D) Infinite
Answer:
(D) Infinite
Explanation:
The reaction will be 100% complete only after infinite time.
![]()
Class 12 Chemistry Ch 4 MCQ Question 13.
Consider a first order gas phase decomposition reaction given below:
A(g) → B(g) + C(g) The initial pressure of the system before decomposition of A was ‘pi‘. After lapse of time ‘f, total pressure of the system increased by x units and became ‘pt‘. The rate constant k for the reaction is given as:
(A) k = \(\frac{2.303}{t} \log \frac{p_{i}}{p_{i}-x}\)
(B) k = \(\frac{2.303}{t} \log \frac{p_{i}}{2 p_{i}-p_{t}}\)
(C) k = \(\frac{2.303}{t} \log \frac{p_{i}}{2 p_{i}+p_{t}}\)
(D) K = \(\frac{2.303}{t} \log \frac{p_{i}}{p_{i}+x}\)
Answer:
(B) k = \(\frac{2.303}{t} \log \frac{p_{i}}{2 p_{i}-p_{t}}\)
Explanation:
Let us consider a first order gas phase decomposition reaction:
A(g) → B(g) + C(g)
The initial pressure of the system before decomposition of A is ‘Pi‘ After lapse of time ‘t’, total pressure of the system increased by x units and became ‘Pt‘.
Hence, the pressure of A decreased by x atom.
Initial pressure: Pi, atom 0 0
Pressure after time t: (P,-.v) x atm x atm
Pt = (Pi – x) + x + x
= Pt + x atm.
x = Pt – Pi
= Pi – (Pt + Pi)
PA = 2Pi – Pt
k = \(\frac{2.303}{t} \log \frac{[A]_{0}}{[A]}\)
= \(\frac{2.303}{t} \log \frac{P_{i}}{2 P_{i}-P_{t}}\)
Class 12 Chemical Kinetics MCQ Question 14.
For the reaction A → B, the rate of reaction becomes three times when the concentration of A is increased by nine times. What is the order of reaction ?
(A) 1
(B) 2
(C) 1/2
(D) 0
Answer:
(C) 1/2
Explanation:
A → B
Rate of reaction r →[A]n ……(i)
concentration of A is increased by nine limes, then rale of reaction becomes three times,
r’= 3r
A’ = 9 A
r’ ∝[Ar]n
3r’ ∝ [9A]n ………..(ii)
From eQuestion (i) and (ii)
\(\frac{r}{3 r}=\frac{[\mathrm{A}]^{n}}{[9 \mathrm{~A}]^{n}}\)
\(\frac{1}{3}=\left(\frac{1}{3}\right)^{2 n}\)
1 = 2n
n = 1/2
Order of reaction = [1/2 ]
![]()
Chemistry MCQ Class 12 Chapter 4 Question 15.
A first-order reaction is 50% completed in 1.26 x 1014 s. How much time would it take for 100% completion?
(A) 1.26 x 1015 s
(B) 2.52 x 1014 s
(C) 2.52 x 1028 s
(D) Infinite
Answer:
(D) Infinite
Explanation:
The reaction will be 100%- complete only after infinite time.
MCQ Chemical Kinetics Chapter 4 Class 12 Question 16.
The value of rate constant of a pseudo-first- order reaction:
(A) depends on the concentration of reactants present in small amount.
(B) depends on the concentration of reactants present in excess.
(C) is independent of the concentration of reactants.
(D) depends only on temperature.
Answer:
(B) depends on the concentration of reactants present in excess.
Explanation:
Rale constant of a pseudo-first- order reaction depends on the concentration of reactants present in excess.
MCQ Of Chemical Kinetics Chapter 4 Question 17.
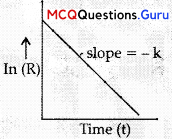
The slope in the plot of ln [R] vs. time gives
(A) +k
(B) \(\frac{+k}{2.303}\)
(C) -k
(D) \(\frac{-k}{2.303}\) (where [R] is the final concentration of reactant.)
Answer:
(C) -k
Explanation:
Assertion And Reason Based MCQs
Directions: In the following questions, A statement of Assertion (A) is followed by a statement of Reason (R). Mark the correct choice as.
(A) BothAandRare true and R is the correct explanation of A
(B) Both A and R are true but R is NOT the correct explanation of A
(C) A is true but R is false
(D) A is false and R is true
MCQs On Chemical Kinetics Chapter 4 Class 12 Question 1.
Assertion (A): Rate of reaction doubles when concentration of reactant is doubled if it is a first order reaction.
Reason (R): Rate constant also doubles.
Answer:
(C) A is true but R is false
Explanation: 1-or first order reaction
Rate – k[A1]
[A2] – [2A1 ]
Rate2 k|2A1 |
Rate2 = k x 2Rate1
For a given reaction, rate constant is constant and I independent of the concentration of reactant.
![]()
Chapter 4 Chemistry Class 12 MCQs Question 2.
Assertion (A): The rate of reaction increases with increase in temperature.
Reason (R): The reactant molecules collide less fre-quently.
Answer:
(C) A is true but R is false
Explanation:
As the temperature of a reaction „is increased, the rate of the reaction increases because Lhe reactant molecules collide more frequently and with greater energy per collision.
Question 3.
Assertion (A): Dust particles suspended in the air inside unheated gain electrons can sometimes react explosively.
Reason (R): The dust particles have large surface area for the reaction.
Answer:
(A) BothAandRare true and R is the correct explanation of A
Explanation:
Since llit- dust par tides have large surface area fur the reacliun su these particles suspended in the air inside unhealed gains electrons can sometimes react explosively.
Question 4.
Assertion (A): Elementary reactions have same value of order and molecularity.
Reason (R): Molecularity is the number of molecules that participate in the reaction, while order is an experimental quantity.
Answer:
(B) Both A and R are true but R is NOT the correct explanation of A
Explanation:
An elementary reaction is a chemical reaction in which one or more chemical species react directly lo form products in a single reaction step and wiLh a single transition stale. Elementary reactions have same value of order and molecularity. Molecularity is the number of molecules that participate in the reaction, while order is an experimental quantity. :
Question 5.
Assertion (A): Hydrolysis of an ester follows first order kinetics.
Reason (R): Concentration of water remains nearly constant during the course of the reaction.
Answer:
(A) BothAandRare true and R is the correct explanation of A
Explanation:
CH3COOC2H5-H2O(ester) →C2H5COOH + C2H5OH
Hydrolysis of an ester follows first order kinetics as [H2O| remains nearly constant during the course of the reaction. It is pseudo first order reaction.
![]()
Question 6.
Assertion (A): For complex reactions molecularity and order are not same.
Reason (R): Order of reaction may be zero.
Answer:
(B) Both A and R are true but R is NOT the correct explanation of A
Explanation:
For a complex reaction,
Order of overall reaction = molecularity of slowest step As rate of overall reaction depends upon total J number of molecules involved in slowest step f of the reaction. lence, for complex reaction. molecularity and order are not same.
Question 7.
Assertion (A): Order of the reaction can be zero or fractional.
Reason (R): We cannot determine order from balanced chemical equation.
Answer:
(B) Both A and R are true but R is NOT the correct explanation of A
Explanation:
Order of a reaction may be zero fractional. It can be determined through the rate f law expression by sum of power ol reactants.
Question 8.
Assertion (A): For a first order reaction, half-life period is independent of initial concentration of the reacting species.
Reason (R): The half-life of a reaction is the time in which the reactant concentration is reduced to one half of its initial concentration.
Answer:
(B) Both A and R are true but R is NOT the correct explanation of A
Explanation:
For a first order reaction, half-life period is independent of initial concentration of the reading species and is calculated from the rate constant or vice versa.
Case-Based MCQs
I. Read the passage given below and answer the following questions:
The rate of a reaction, which may also be called its velocity or speed, can be defined with relation to the concentration of any of the reacting substances, or to that of any product of the reaction. If the species chosen is a reactant which has a concentration c at time t the rate is – dc’dt, while the rate with reference to a product having a concentration x at time t is dWdt. Any concentration units may be used for expressing the rate; thus, if moles per liter are employed for concentration and seconds for the time, the units for the rate are moles litre1sec1.
![]()
For gas reactions pressure units are sometimes used in place of concentrations, so that legitimate units for the rate would be (mm. Hg) sec’ and atm. sec The order of a reaction concerns the dependence of the rate upon the concentrations of reacting substances; thus, if the rate is found experimentally to be proportional to the ath power of the concentration of one of the reactants A, to the f3 power of the concentration of a second reactant B, and so forth, via.,
rate = k CAα CAβ
the over-all order of the reaction is simply n = α + β + ………..(2)
Such a reaction is said to be of the ath order with respect to the substance A, the pth order with respect to B. In the following questions, a statement of Assertion followed by a statement of Reason is given. Choose the correct answer out of the following choices on the basis of the above passage.
(A) Assertion and reason both are correct statements and reason is correct explanation for assertion.
(B) Assertion and reason both are correct statements but reason is not correct explanation for assertion.
(C) Assertion is correct statement but reason is wrong statement.
(D) Assertion is wrong statement but reason is correct statement.
Question 1.
Assertion: Rate of reaction is a measure of change in concentration of reactant with respect to time.
Reason: Rate of reaction is a measure of change in concentration of product with respect to time.
Answer:
(B) Assertion and reason both are correct statements but reason is not correct explanation for assertion.
![]()
Question 2.
Assertion: For a reaction: P + 2Q → Products, Rate = k \([\mathrm{P}]^{1 / 2}[\mathrm{Q}]^{1}\) so the order of reaction is 1.5
Reason: Order of reaction is the sum of stoichiometric coefficients of the reactants.
Answer:
(C) Assertion is correct statement but reason is wrong statement.
Question 3.
Assertion: The unit of k is independent of order of reaction.
Reason: The unit of k is moles L-1s-1.
Answer:
(D) Assertion is wrong statement but reason is correct statement.
Question 4.
Assertion: Reactions can occur at different speeds.
Reason: Rate of reaction is also called speed of reaction.
Answer:
(B) Assertion and reason both are correct statements but reason is not correct explanation for assertion.
II. Read the passage given below and answer the following questions:
The rate of the reaction is proportional to the concentration of the reactant. Hydrogenation of ethene results in the formation of ethane. The rate constant, k for the reaction was found to be 2.5 x 1015s-1. The concentration of the reactant reduces to one-third of the initial concentration in 5 minutes. The following questions are multiple choice questions. Choose the most appropriate answer:
Question 1.
Find the order of reaction:
(A) Zero order
(B) First order
(C) Second order
(D) Fractional order
Answer:
(B) First order
Explanation:
Since the rate of the nuition proportional to the concentration for the reactant i.e. ethene so, it is first order reaction.
Question 2.
The rate law equation is:
(A) Rate = k [C2H6]
(B) Rate = k [C2H4]2
(C) Rate = k [C2H4]
(D) Rate = k [C2H4]2
Answer:
(C) Rate = k [C2H4]
Explanation:

Rate law equation,
Rate = K [C2H4]
![]()
Question 3.
The half-life for the reaction is:
(A) 2.772 x 1024 s
(B) 2.772 x 1012s
(C) 1.386 x 10-24 s
(D) 1.386 x 10-12 s
Answer:
(A) 2.772 x 1024 s
Explanation:
For first order reaction,
\(t_{1 / 2}=\frac{0.693}{K}\)
= \(\frac{0.693}{2.5 \times 10^{-15} \mathrm{~s}^{-1}}\)
= 2.772 x 10-24s
Question 4.
The rate constant of the reaction after 5 minutes is:
(A) 0.4290 min-1
(B) 0.1297 min-1
(C) 0.2197 min-1
(D) 0.6591 min-1
Answer:
(C) 0.2197 min-1
Explanation: t = 5 min
\(\frac{[R]_{0}}{[R]}\) = 3
For first order reaction,
K = \(\frac{2.303}{t} \log \frac{[\mathrm{R}]_{0}}{[\mathrm{R}]}\)
= \(\frac{2.303}{5} \log 3\)
= \(\frac{2.303}{5} \times 0.4771\)
= 0.2197 min-1
OR
The slope of the curve in the reaction is:
(A) K
(B) -K
(C) 2K
(D) -2K
Answer:
(B) -K
Explanation:
Slope = -k
III. Concentration dependence of rate is called differential rate equation. Integrated differential equations give relation between directly measured experimental data i.e. concentration at different times and rate constant. The integrated rate equations are different for the reactions of different reaction orders. The first-order reaction has a rate constant 1.15 x 10-3 3s-1
The following questions are multiple choice questions. Choose the most appropriate answer:
Question 1.
How long will 5g of this reactant take to reduce to 3g?
(A) 222.189 s
(B) 444.379 s
(C) 111.095 s
(D) 888.789 s
Answer:
(B) 444.379 s
Explanation:
Initial amount = 5 g
Final concentration = 3 g
Rate constant = 1.15 x 10-3 s-1
We know that for a First order reaction
t = \(\frac{2.303}{k} \log \frac{\left[R_{\text {intal }}\right]}{\left[R_{\text {final }}\right]}\)
= \(\frac{2.303}{1.15 \times 10^{-3}} \log \frac{5}{3}\)
= \(\frac{2.303}{1.15 \times 10^{-3}} \times 0.2219\)
= 444.379 s
![]()
Question 2.
When the rate constant has same units as the rate of reaction, the order of the reaction is:
(A) Zero order
(B) First order
(C) Second order
(D) Fractional order
Answer:
(A) Zero order
Explanation:
In case of zero order reaction, the rate constant has same units as the rate of reaction.
r = k [A]0
r = K
Unit of rate = mol L-1 s-1
Unit of k = mol L-1 s-1
Question 3.
Under which condition a bimolecular reaction is kinetically first order reaction:
(A) When two reactants are involved.
(B) When one of the reactants is in excess.
(C) When one of the reactants does not involve in reaction.
(D) None of these.
Answer:
(B) When one of the reactants is in excess.
Explanation:
When one of the reactants is in excess, a bimolecular reaction is kinetically first order reaction.
Question 4.
For a reaction,
A + H2O → B
Rate ∝ [A]
The order of the reaction is:
(A) Zero order
(B) Fractional order
(C) Pseudo first order
(D) Second order
Answer:
(C) Pseudo first order
Explanation:
Slope = A + H2O → B
r ∝ [A] (∴ [H2O] = excess)
It is called pseudo first order reaction.
OR
The integrated rate equation for rate constant of a first order reaction is:
(A) \(\mathrm{k}=\frac{2.303}{\mathrm{t}} \log \frac{[\mathrm{R}]_{0}}{[\mathrm{R}]}\)
(B) \(\mathrm{k}=\frac{1}{\mathrm{t}}\left[\frac{1}{[\mathrm{R}]}-\frac{1}{[\mathrm{R}]_{0}}\right]\)
(C) \(k=\frac{1}{2 t}\left[\frac{1}{[R]^{2}}-\frac{1}{[R]_{0}^{2}}\right]\)
(D) None of these