NCERT Solutions for Class 9 Science Chapter 1 Matter in Our Surroundings
These Solutions are part of NCERT Solutions for Class 9 Science. Here we have given NCERT Solutions for Class 9 Science Chapter 1 Matter in Our Surroundings. LearnInsta.com provides you the Free PDF download of NCERT Solutions for Class 9 Science (Chemistry) Chapter 1 – Matter in Our Surroundings solved by Expert Teachers as per NCERT (CBSE) Book guidelines. All Chapter 1 – Matter in Our Surroundings Exercise Questions with Solutions to help you to revise complete Syllabus and Score More marks.
More Resources
- NCERT Solutions for Class 9 Science
- HOTS Questions for Class 9 Science
- Value Based Questions in Science for Class 9
- NCERT Exemplar Solutions for Class 9 Science
- Previous Year Question Papers for CBSE Class 9 Science
NCERT IN TEXT PROBLEMS
IN TEXT QUESTIONS
Question 1.
Which of the following are matter ?
Chair, air, love, smell, hate, almonds, thought, cold, cold drink, smell of perfume.
Answer:
Chair, air, smell, almonds, cold drink, smell of perfume are matter. Please note that the smell whether pleasant or foul is due to the presence of some particles in air. It is therefore, a matter. Love, hate, thought and cold are simply feeling. They do not represent matter.
Question 2.
The smell of hot sizzling food reaches us several metres away. However, it is not so in case the food is cold. Explain.
Answer:
When food is sizzling hot, it releases the vapours of its contents. Since the kinetic energy of the particles is very
high in the vapour state, these particles readily mix up with the particles of air. They can reach us even at a distance of several metres. However, when the food is cold, the vapours released will be comparatively less. Moreover, their kinetic energy is also very small. Under these conditions, one has to come quite close in order to smell the contents of the food.
Question 3.
A diver is able to cut through water in a swimming pool. Which property of matter does this observation show ?
(CBSE 2013)
Answer:
The observation explains the following properties of water (liquid state of matter) or any other liquid.
- The inter particle forces of attraction are not very strong in water.
- The inter particle spaces are somewhat large in water.
- All these properties associated with the liquid state or water enable the diver to cut through water in a swimming pool.
Question 4.
What are the characteristics of the particles of matter ?
Answer:
Characteristics of the Particles of Matter
We have studied that matter is composed by particles and there are interparticle spaces as well. Let us brief by study the main characteristics of these particles supported by some activities.
- Particle Size is Extremely Small
- Particles are in a State of Motion
- Inter Particle Attractions Keep Particles Close
- Effect of Temperature on Particle Motion
Question 5.
The mass per unit volume of a substance is known as density (density = mass/volume). Arrange the following in order of increasing density :
Air, exhaust from chimneys, honey, water, chalk, cotton and iron.
Answer:
The increasing order of density for the given substances is :
Air, exhaust from chimneys, cotton, water, honey, chalk, iron.
Actually, the density of a substance depends upon the number of particles per unit volume as well as upon their mass. The number of the particles is related to their size as well as the attractive forces among them. Keeping this in view, the increasing order of density is as given above.
Question 6.
Tabulate the differences in the characteristics of the three states of matter.
Answer:
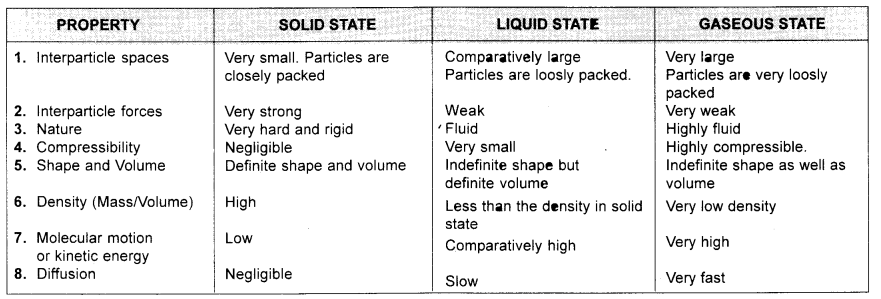
Question 7.
Comment on the following :
rigidity, compressibility, fluidity, filling of a gas in cylinder, shape, kinetic enegy and density.
Answer:
Rigidity: Solids are known for their hardness and rigid nature.
Compressibility: Actually, the constituent particles are so closely packed in a solid that they either do not come closer or do so only slightly when a high pressure is applied. However, there are some exceptions. For example, a sponge made from some foam or rubber material can be easily compressed.
Fluidity: We have seen that rigidity is maximum in the solid state and fluidity or particle motion is negligible. In the liquid state of a substance, both these characters are different. The liquids are less rigid than the solids and the molecular motion is also comparatively more.
Filling of a Gas in Cylinder: All of us are quite familiar with a cooking gas cylinder which contains in it liquefied petroleum gas, often called L.P.G. It is a mixture of different gases such as propane, butane etc. These are so highly compressed that they are in the liquefied form. When the regulator is opened, the liquid escapes from the nozzle of the cylinder into a space where pressure is very less.
Shape: Solids generally have fixed shapes. They donot change their shapes even when put in different containers. For example, blue crystals of copper sulphate have needle like shape which they retain whether kept in a beaker or in a china dish or placed on the palm of our hand.
Kinetic Enegy: The kinetic energy of the particles in the liquid state of a substance is more than in solid state. It further increases with the rise in temperature.
Density: The density of the gas (mass/volume) is very small and the gases are therefore, light.
Question 8.
Why does a gas fill completely the vessel in which it is kept ?
Answer:
This happens because of fast diffusion of the particles in a gas. The number of vacant spaces or voids in the gaseous state is very large. This means that the particles of a gas move at a very fast speed. They readily fill completely the vessel in which the gas is kept. Thus, the volume of the gas is the same as that of the vessel.
Question 9.
A gas exerts pressure on the walls of the container. Assign reason. (CBSE 2012, 2013)
Answer:
The molecules in a gas have large kinetic energy. They strike the walls of the container with certain force and impart momentum to them. The force per unit area or momentum is responsible for the pressure of the gas.
Question 10.
Why should we call a wooden table a solid ? (CBSE 2012, 2013)
Answer:
A wooden table should be called a solid because it matches the characteristics of the solid state. For example,
- It is very hard and rigid.
- Its shape cannot be changed by altering temperature or pressure. .
- It is quite heavy which means high density.
- There is no movement of the constituent particles present.
Question 11.
We can easily move our hand in air but to do so the same through a solid block of wood, we need a ‘Karate expert’. Explain.
Answer:
In air, the interparticle spaces are very large in number and the interparticle forces are quite weak. These can be easily overcome. That is why our hand can move in air. These spaces help in moving our hand in air, But in a solid block, the constituent particles are quite close and the interparticle forces are very strong. In case, one has to move his hand through a solid, it will be extremely difficult. Only a karate expert may do so.
Question 12.
Liquids generally have low density as compared to solids. But you must have observed that ice floats on water. Find out why ?
Answer:
Ice (solid state) is expected to be heavier than water (liquid state). But it is lighter and floats over water. Actually, ice has a cage like structure which means that vacant spaces are left when H2O molecules are linked in ice. The number of these spaces are comparatively less in water. In other words, we can also say that the structure of ice is more porous than that of water. Therefore, water is dense as compared to ice or ice floats over water.
Question 13.
Convert the following temperatures to Celsius scale :
(a) 300 K
(b) 573 K.
Answer:
(a) (300 – 273) = 27°C
(b) (573 – 273) = 300°C.
Question 14.
What is the physical state of water at : (a) 250°C (b) 100°C ?
Answer:
Boiling point temperature of water is 100°C. Above this temperature (250°C), water exists in the gaseous state i.e., as vapours or as steam. However, at 100°C, both liquid and gaseous states are present. Actually, at the boiling point temperature, both the liquid and gaseous states of a substance co-exist. These are in a state of equilibrium. We may conclude that at 100°C, both liquid water and vapours are present but at 250°C we have only vapours or steam and no liquid.
Question 15.
For any substance, why does the temperature remain constant during the change of state ?
Answer:
Once the change of state of a substance begins or starts, the energy which is now supplied is being used up as latent heat. It means that it does not increase the kinetic energy of the particles and is used up only to overcome the inter-particle forces in that particular state and to bring about a change in state. Therefore, the temperature becomes constant.
Question 16.
Suggest a method to liquefy atmospheric gases.
Answer:
In order to liquefy a gas, the constituent particles or molecules have to be brought closer. The atmospheric gases can be liquefied either by increasing pressure or by decreasing temperature.
Question 17.
A cooler is quite effective on a hot and dry day. Explain.
Answer:
Under the conditions, the humidity level in the atmosphere is quite low and the evaporation rate of water is expected to be high. Since cooling is caused during evaporation, the air which escapes from the cooler is compartively cold under these conditions. Therefore, is quite effective on a hot and dry day.
Question 18.
How does water kept in an earthen pot become quite cold during summer ?
Answer:
The earthen pot is full of small pores. Water present in these pores has a tendency to escape at a fast rate during summer. The escaping molecules of water appear as water vapours and evaporate. Since cooling is caused in evaporation, the temperature of the water inside the earthen pot gets considerably lowered and it becomes cold.
Question 19.
When we pour some acetone or perfume on our palm, we get a cooling sensation. Assign reason.
Answer:
Both acetone and perfume are low boiling liquids. When any of them is poured on the palm, it readily changes into vapours or evaporates. For this, it needs some energy which is taken from the palm. The temperature of the palm gets lowered and we get a cooling sensation.
Question 20.
Why can we sip hot tea from a saucer faster than from a cup ?
Answer:
The phenomenon of change of liquid to the vapour state at any temperature below the boiling point of the liquid.
In a liquid, the particles or molecules experience mutual forces of attraction. However, these are not stationary and have some kinetic energy at all temperatures. The particles of a liquid are also colliding with one another and exchanging energy during the collisions. Above the liquid surface, atmosphere or air is present which is a mixture of several gases. The particles of the liquid present on the surface have a tendency to come out from the surface so that they may acquire more freedom to move and become part of the atmosphere. This is also known as randomness. To overcome the interparticle forces of attraction, they need some energy which they take up from the rest of the particles or molecules of the liquid. As a result, their temperature gets lowered and cooling is caused.
NCERT END EXERCISE
Question 1.
Convert the following temperatures to Celsius scale ?
(a) 293 K
(b) 470 K.
Answer:
(a) (293 – 273) = 20°C
(b) (470 – 273) = 197°C
Question 2.
Convert the following temperatures to Kelvin scale
(a) 25°C
(b) 373°C. (CBSE 2014)
Answer:
(a) (25 + 273) = 298 K
(b) (373 + 273)’= 646 K.
Question 3.
Give reasons for the following observations :
(a) Naphthalene balls disappear with time without leaving any solid (CBSE 2011, 2013)
(b) We can get the smell of perfume sitting several metres away. (CBSE 2013)
Answer:
(a) Naphthalene has a tendency to sublime i.e. it changes directly to the gaseous state. Therefore, the size of the naphthalene balls slowly decreases and ultimately they disappear. No solid residue is left.
(b) A perfume is actually a mixture of number of pleasant smelling vapours. They diffuse quite fast and can reach a person who may be even at several metres away from a person who has used perfume.
Question 4.
Arrange the following substances in increasing order of attraction between the particles : water, sugar, oxygen.
(CBSE 2012)
Answer:
The three substances differ in their physical state at normal temperature. Oxygen is a gas, water a liquid while sugar is a crystalline solid. Keeping this is mind, the increasing order of attraction between the particles is : oxygen < water < sugar.
Question 5.
What is the physical state of water at
(a) 25°C (b) 0°C (c) 100°C ? [CBSE 2014]
Answer:
(a) At 25°C, water is in the liquid state (b) At 0°C, water can exist both in the solid state (ice) and in liquid state. This temperature represents the melting point of ice and freezing point of water (c) At 100°C, water can be present both in the liquid and vapour states. This temperature corresponds to the boiling point of water and liquefication temperature of steam.
Question 6.
Give two reasons to justify that :
- water at room temperature is a liquid
- an iron almirah is a solid at room temperature. (CBSE 2012, 2013)
Answer:
- Water is a liquid at room temperature (25°C) due to the following reasons :
- When placed in a beaker, its level cannot be changed on pressing.
- It can take the shape of any container in which it is placed.
- An iron almirah is a solid due to following reasons :
- Its shape does not change when pressed. This means that it is hard and rigid.
- It is very heavy. This means that its density is very high.
Question 7.
Ice at 273 K causes more cooling than water at the same temperature. Explain. (CBSE 2015)
Answer:
Ice (solid state) has extra energy in the form of latent heat of fusion (335 kj kg-1) as compared to water. When ice is to melt, it takes energy from the surroundings to overcome this latent heat. The temperature of the surroundings gets lowered or cooling is caused. Since water is already in the liquid state, it will hardly take up any energy from the surroundings.
Question 8.
Why does steam produce more severe burns on the skin as compared to boiling water ? (CBSE 2015)
Answer:
Steam is formed when water at its boiling point temperature of 100°C (373 K) absorbs latent heat of vaporisation. Therefore, steam has more energy than boiling water. On account of this, steam produces more severe burns on the skin as compared to boiling water.
Question 9.
Name A, B, C, D, E and F in the following diagram showing change of state. (CBSE 2015)
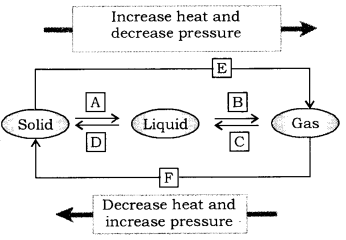
Answer:
[A] = Fusion(melting), [B]=Vaporisation , [C] = Condensation(Liquefaction),
[D] = Solidification, [E] = (freezing), Sublimation, [F] = Solidification of gaseous state.
VERY SHORT ANSWER QUESTIONS
Question 1.
What is common in the three states of matter ?
Answer:
All of them occupy space and have mass.
Question 2.
Can we regard high fever as matter ?
Answer:
Fiigh fever is a feeling only. It cannot be regarded as matter.
Question 3.
Why do not solids possess fluidity ?
Answer:
Fluidity means tendency to flow. The constituents in the solids are very closely packed and interparticle forces are quite strong. Therefore, solids have hardly any fluidity.
Question 4.
A certain substance ‘A’ cannot be compressed but takes up the shape of any container in which it is placed. What will you regard its physical state ?
Answer:
The physical state of the substance ‘A’ is a liquid.
Question 5.
Why do substances undergo change in physical state ?
Answer:
Substances undergo change in physical state because both interparticle spaces and interparticle forces can be changed by changing the conditions of temperature and pressure.
Question 6.
What is the pressure at sea level ?
Answer:
The pressure at sea level is regarded as 1 atmosphere or 760 mm.
Question 7.
Are the melting point temperature of the solid state and the freezing point temperature of the liquid state of a substance different ?
Answer:
No, these are the same. For example, melting point of ice and freezing point of water are both 0°C or 273 K.
Question 8.
Why are gases highly compressible ?
Answer:
Because the interparticle empty spaces are very large. When a gas is compressed, these spaces decrease. The particles or molecules of gas come closer.
Question 9.
A substance is in liquid state at room temperature and changes into gaseous state upon heating. What will you call its gaseous state ?
Answer:
The gaseous state of the substance is regarded as vapours.
Question 10.
When a crystal of copper sulphate is placed at the bottom of a beaker containing water, the colour of water slowly becomes blue, why ?
Answer:
The crystal breaks into fine particles which form copper ions (Cu2+ ions) in solution. As these ions (blue in colour) spread, the colour of water slowly becomes blue.
Question 11.
Why do solids generally lack the property of diffusion ?
Answer:
This is because of the absence of translatory motion in the solid state since the particles are very closely packed.
Question 12.
Do we represent the temperature on kelvin scale by the letter ‘k’ or ‘K’ ?
Answer:
The temperature on Kelvin scale is represented by the letter K (capital letter).
Question 13.
The boiling point of ethyl alcohol is 78°C. What is the corresponding temperature on kelvin scale ?
Answer:
Temperature on kelvin scale = 78 + 273 = 351 K
Question 14.
When a solid starts melting, its temperature does not rise till whole of it has melted. Explain.
Answer:
The heat energy which is now being supplied is used up to bring a change in physical state only. It is known as latent heat of fusion.
Question 15.
A substance upon heating directly changes into gaseous state. What is this change called ?
Answer:
It is known as sublimation.
Question 16.
Define gaseous state of a substance.
Answer:
A substance is said to be in the gaseous state if under normal pressure, its boiling point is below the room temperature. In other words, at room temperature the substance is in the gaseous state.
Question 17.
Represent freezing and boiling temperatures of water in Celsius as well as in Kelvin scales. (CBSE 2012)
Answer:
Freezing point temperature of water : 0°C or 273 K Boiling point temperature of water : 100°C or 373 K.
Question 18.
Does the evaporation of a liquid occur only at a fixed temperature ?
Answer:
No, the evaporation of a liquid occurs at all temperatures.
Question 19.
What is the name given to the process when the vapours of a liquid get cooled ?
Answer:
The process is known as condensation.
Question 20.
Does the temperature of liquid increase further when it starts boiling ?
Answer:
No, it does not because the energy now supplied is used up as latent heat of vaporisation only.
Question 21.
Why do we sweat more in a humid day ?
Answer:
In a humid day, the air around us has already high percentage of water vapours. Therefore, the water coming from the skin gets less opportunity to change into vapours and remains sticking to our body. We therefore, sweat more on a humid day.
Question 22.
How does pressure help in the liquefication of a gas ?
Answer:
Increase in pressure helps in the liquefication of a gas. The particles or molecules of a gas come closer and closer as the pressure is being increased gradually. They ultimately condense and as a result, the gas liquefies or changes into the liquid state.
Question 23.
What is meant by saying that latent heat of vaporisation of water to 226 kj/kg-1.
Answer:
This means that when one kg of water at its boiling point temperature changes into steam, 226 kj of heat is absorbed.
Question 24.
In which state of matter
- Intermolecular forces are the maximum,
- Diffusion is the least
- Intermolecular spaces are the maximum ?
Answer:
- Solid state
- Solid state
- Gaseous state.
Question 25.
Latest heat of vaporisation of two liquids A and B are 100 J/kg and 150 J/kg respectively. Which one can produce more cooling effect ?
Answer:
The liquid B can cause more cooling effect than the liquid A because it absorbs more heat from the surrounding during the change of state from liquid to the vapours.
Question 26.
What temperature in Kelvin scale is equal to 50°C ?
Answer:
50°C = 50 + 273 = 323 K.
Question 27.
Why does a balloon inflate when air is blown into it ?
Answer:
On blowing air into a balloon, the volume of air inside increases. As a result, balloon inflates.
Question 28.
Write full form of
- C.N.G.
- L.P.G.
Answer:
- Compressed natural gas
- Liquid petroleum gas.
Question 29.
Melting points of three solids X, Y and Z are 298K, 314K and 398K respectively. Arrange these in increasing order of inter particle forces of attraction.
Answer:
Please remember that greater the melting point temperature of a solid, more is the magnitude of interparticle forces. The correct is X < Y < Z.
Question 30.
What happens to the heat energy supplied to a solid once it starts melting ?
Answer:
The heat energy is supplied as latent heat of fusion. There is no change in the temperature till the entire solid has melted. .
Question 31.
You are provided with a fine white coloured powder which is either sugar or salt. How will you identify it without testing ?
Answer:
We can identify them by taste only. Sugar tastes sweet whereas common salt as we all know, has a bitter taste.
Question 32.
Name the term used for the following : (CBSE 2014)
- Conversion of vapours to solid
- Conversion of solid to liquid
- Conversion of vapours to liquid.
Answer:
- Freezing or solidification
- Melting
- Condensation.
SHORT ANSWER QUESTIONS
Question 33.
Assign reasons for the following :
Why do wet clothes do not dry easily on a rainy day. (CBSE 2016)
Answer:
In a rainy day, the humidity level in the atmosphere is already high. As a result, it will be quite difficult for the moisture associated with wet clothes to change into vapours. Therefore, they will remain wet.
Question 34.
Why are gases highly compressible while solids are almost incompressible ?
Answer:
In the gases, the inter particle or inter molecular spaces are very large. These can be decreased to a large extent on applying pressure. Therefore, the gases are highly compressible, very small and cannot be decreased any more on applying pressure, incompressible.
Question 35.
Arrange in the order indicated for solid, liquid and gas.
- effect of pressure – increasing order
- empty spaces in the particles – decreasing order
- tendency to flow – decreasing order
- thermal expansion – increasing order.
Answer:
- solid, liquid, gas
- gas, liquid, solid
- gas, liquid, solid
- solid, liquid, gas.
Question 36.
Name the state of matter that
- has definite mass, volume and shape
- has no definite volume and shape
- has minimum inter particle attraction
- has maximum inter particle attraction.
Answer:
- solid state
- gaseous state
- gaseous state
- solid state
Question 37.
(a) Butter is generally wrapped in wet cloth during summer if no refrigerator is available. Explain.
(CBSE 2015)
(b) The Latent heat of vaporisation of steam is more than that of the boiling water. Explain.
Answer:
(a) In summer, the weather is a quite hot. As a result, water present in wet cloth readily evaporates. Since cooling is caused during evaporation, the temperature of butter gets lowered. This checks the rancidity of butter or it does not give any foul odour.
(b) When boiling water changes into steam, it absorbs a certain amount of heat energy. This shows that the latent heat of vaporisation of steam is more than that of boiling water.
Question 38.
Which of the following factor(s) is (are) responsible for the change of state of solid carbon dioxide into vapours ?
(a) Increase in pressure
(b) Decrease in presssure
(c) Increase in temperature
(d) Decrease in temperature.
Answer:
The change of state can take place by decreasing the pressure and increasing the temperature.
Question 39.
How will you justify that ice, water and steam are not different substances but different states of the same substance ?
Answer:
When ice (solid state) is heated, it melts and changes to water (liquid state). When water is boiled, it is converted to steam (gaseous state). The process can be reversed upon cooling. This justifies that these are the states of a substance. In fact all the three states are chemically the same with the formula H2O.
Question 40.
Name the process associated with the following :
(a) Dry ice is kept at room temperature and under one atmosphere pressure.
(b) A drop of ink placed on the surface of water contained in a glass spreads throughout water.
(c) A crystal of potassium permanganate is put in water contained in a beaker and stirred with a glass rod.
(d) Milk is churned to seperate cream from it.
(e) Settling of sand when a mixture of sand and water is left undisturbed for sometime.
Answer:
(a) Sublimation
(b) Diffusion
(c) Dissolution/diffusion
(d) Centrifugation
(e) Sedimentation.
Question 41.
(a) Solids are generally very heavy while gases are light. Explain.
(b) Carbon dioxide gas is heavier than both nitrogen and oxygen. Why does not it form lower layer in the atmosphere ?
Answer:
(a) In the solids, the particles are very closely packed. As a result, the number of particles per unit volume is quite large. Therefore, the solids are normally quite heavy. In the gases, the particles are loosly packed. The number of particles per unit volume is comparatively small. Therefore, gases are light.
(b) The diffusion of a gas is not affected by gravity. This means that carbon dioxide (CO2) remains uniformly mixed in air. Therefore, the gas does not form the lower layer in the atmosphere.
Question 42.
How do surface area and wind speed affect rate of evaporation ? (CBSE 2014)
Answer:
With increase in surface area, more molecules of the liquid get opportunity to come to the surface and change into vapours. Similarly, with the increase in wind speed, the particles of water vapours present in air move away and the air which replaces it is comparitively dry. This will increase the rate of evaporation.
Question 43.
(a) Explain why there is no rise in temperature of a substance when it undergoes a change of state although it is still being heated.
(b) Name one property which is shown by naphthalene and not by sodium chloride.
Answer:
(a) Once the change in state of a substance starts (solid to liquid or liquid to gas), the temperature of the substance does not change. Actually, the heat energy now supplied does not increase the kinetic energy of the constituting particles. It is absorbed either as latent heat of fusion or as latent heat of vaporisation.
(b) Naphthalene undergoes sublimation upon standing/heating and directly changes into vapours. Sodium chloride (common salt) does not undergo sublimation. It melts on strong heating.
Question 44.
The molecules of water have more energy as compared to molecules of ice at same temperature. Justify this statement.
Answer:
When ice is to melt, energy is absorbed to overcome intermolecular forces of attraction. Since the intermolecular forces of attraction decrease, the kinetic energy of molecules in water is more than in ice.
Question 45.
Define (a) Latest heat of fusion, (b) Melting point (c) Fusion.
Answer:
(a) Latent heat of fusion : It is the amount of heat energy which is needed to convert one kg of a solid into the liquid state at its melting point temperature without any rise in temperature under one atmosphere pressure.
(b) Melting point : It is the tempetature at which a solid starts melting or starts changing into the liquid state.
(c) Fusion : The process of the change of the solid state into the liquid state is known as fusion.
Question 46.
Give reasons for the following :
(a) A liquid generally flows easily.
(b) Ice at 0°C appears colder in the mouth than water at 0°C.
(c) Doctors advise to put strips of wet cloth on the forehead of a person having high fever.
Answer:
(a) The intermolecular forces in a liquid are weak. Its molecules are able to slide over one another and liquid generally flows easily.
(b) Latent heat of fusion of ice is quite high. In the mouth, ice absorbs this heat and melts. That is why ice at 0°C gives more cooling sensation in the mouth than water at the same temperature.
(c) As water evaporates from the strips of wet cloth placed on the forehead of a person, it absorbs heat from the body. The body temperature gets lowered and the person gets some relief from fever.
Question 47.
Prachi took 50 mL of water in two beakers at room temperature and added sodium chloride to one beaker while sugar to the other, till no more solute would dissolve. Then she heated the contents of the beakers and added more solutes in them.
(a) Will the amount of salt and sugar that can be dissolved in water at given temperature same ?
(b) What will you expect to happen if she cools the contents of the beakers ? Justify your answer.
Answer:
(a) No, they will be different. Actually sodium chloride (salt) is a crystalline solid while sugar is a molecular solid. They dissolve to different extent. Sodium chloride is more soluble in water compared, to sugar at a given temperature,
(b) Upon cooling, solid solute will slowly start separating from the solution. In general, the solubility of a solid in a liquid increases with the rise in temperature and decreases as the temperature is lowered.
Question 48.
Explain the interconversion of three states of matter in terms of force of attraction and kinetic energy of the molecules.
Answer:
The intermolecular force of attraction is maximum in the solid state and minimum in the gaseous state. Similarly, the kinetic energy of the molecules is minimum in the solid state and maximum in the gaseous state. The change of state can be brought about by either changing the temperature or pressure.
Question 49.
Carbon dioxide was taken in an enclosed cylinder and compressed by applying pressure
(a) Which state of matter will we obtain after completion of the process ?
(b) Name and define this process.
(c) What is the common name of the product obtained in the above process ?
Answer:
(a) Liquid state of matter will be obtained,
(b) The process is known as liqueficadon. It is the change of state from gas to the liquid by increasing pressure or decreasing temperature,
(c) It is known as liquid CO2.
LONG ANSWER QUESTIONS
Question 50.
(a) What is the reason for the existence of the three states of matter ?
(b) What will happen when solid ammonium chloride is heated ?
(c) The room temperature is 25°C. What is the corresponding temperature on the Kelvin scale ?
(d) What happens to the particle motion if the temperature of the gas is increased ?
(e) A substance ‘X’ was highly compressible and could be easily liquified. It could also take up the shape of any container. Predict the nature of the substance. Enlist four properties of this state of matter.
Answer:
(a) The three states of matter differ with respect to the inter particles spaces. These are minimum in the solid state while maximum in the gaseous state,
(b) It will directly change to the vapour state without passing through the liquid state. The process is known as sublimation.
(c) Kelvin temperature (K) = 273 + 25 = 298 K.
(d) With the increase in temperature, the average kinetic energy of the particles increases. As a result, the particle motion increases.
(e) The substance ‘X’ appears to be a gas since the characteristics shown by it resemble those of the gaseous state. For the properties of the gaseous state.
Question 51.
With the help of an activity show that air contains water vapours.
Answer:
In a tumbler made up of steel, take crushed ice. Completely dry it from outside with a towel. Allow the tumbler to stand lor about ten minutes. Drops of water will be seen deposited on the outer walls of the tumbler.
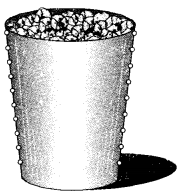
Explanation: Air contains in it a very small percent of water as vapours. These pass into the atmosphere as a result of evaporation which is taking place from water present in ponds, rivers, lakes, oceans etc. When these water vapours present in air come in contact with outer surface of steel tumbler, they condense because of the presence of ice inside it. The drops of water thus formed appear on the outer walls of the tumbler.
Question 52.
Define melting point of a solid. At what temperature on the Kelvin scale does ice melt ? In the experiment to determine the melting point of ice, why does the temperature not rise till all the ice has melted even though heat is supplied continuously ? What is this heat energy called ?
Answer:
As long as ice has not melted, the heat energy supplied increases the kinetic energy of particles (or H2O molecules) which constitute ice. As a result, the temperature rises. Once the process of melting of ice starts, the heat energy is now consumed in bringing about the change in state from solid to liquid. We can also say that the heat energy is used up to overcome the intermolecular forces of attraction present among the H20 molecules in ice. Thus, it is absorbed by ice without changing the temperature. This is known as latent heat which means that it is in hidden form as it is not visible. (In Greek : latent means-hidden) In case of solids, it is known as latent heat of fusion (fusion means melting). It may be defined as :
The amount of heat energy that is needed to convert one kg of a solid into the liquid state at its melting point temperature without any rise in temperature under atmospheric (one atmosphere) pressure.
Question 53.
With the help of a labelled diagram describe an activity to show that particles of matter are very small. Use the following material that has been provided to you. (CBSE 2010, 2015)
Four beakers, three test tubes, dropper, distilled water and few crystals of potassium permanganate.
Answer:
Activity : In four glass beakers marked as 1, 2, 3 and 4, take distilled water. Fill each beaker to about half. With the help of a spatula, add a few crystals of potassium permanganate to the beaker (1). Stir with the spatula and note the colour of the solution. It will be dark purple. Now transfer a small volume of the solution in a test tube and add this solution to the beaker (2). The solution will be still purple but the colour will be lighter as compared to the solution in beaker (1). Repeat the experiment in beaker (3) and also in beaker (4). The purple colour of the solution will not disappear even in beaker (4) although it will be very very light.
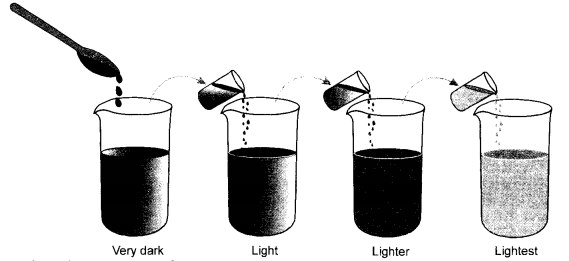
The activity shows that the particles of a matter are very small in size and become even smaller. Flowever, they donot disappear.
Question 54.
Account for the following :
(a) The temperature of water remains constant during boiling.
(b) Evaporation is a surface phenomenon.
(c) The spaces between constituent particles is maximum in the gaseous state.
Answer:
(a) Once a liquid starts boiling, there is no increase in average kinetic energy upon further heating. The heat now supplied is used to overcome intermolecular forces only. Therefore, the temperature of water remains constant during boiling.
(b) Molecules or particles of a liquid present on the surface have an urge or desire to escape from the surface as vapours in order to increase their free movement or randomness. This is known as evaporation. Thus, evaporation is a surface phenomenon. More the surface area available more will be the rate of evaporation.
(c) In the gaseous state of matter the interparticle forces are the least. Therefore, interparticle spaces are the maximum.
NCERT Solutions for Class 9 Science Chapter 1 Matter in Our Surroundings
Hope given NCERT Solutions for Class 9 Science Chapter 1 are helpful to complete your science homework.
If you have any doubts, please comment below. Learn Insta try to provide online science tutoring for you.