Amines Class 12 MCQs Questions with Answers
Amines MCQ Class 12 Chapter 13 Question 1.
CH3CONH2 on reaction with NaOH and Br2 in alcoholic medium gives:
(A) CH3CH2NH2
(B) CH3CH2Br
(C) CH3NH2
(D) CH3COONa
Answer:
(C) CH3NH2
Explanation:
![]()
Amines Class 12 MCQ Chapter 13 Question 2.
Propanamide on reaction with bromine in aqueous NaOH gives:
(A) Propanamine
(B) Ethanamine
(C) N-Methyl ethanamine
(D) Propanenitrile
OR
IUPAC name of product formed by reaction of methyl amine with two moles of ethyl chloride
(A) N,N-Dimethylethanamine
(B) N,N-Diethylmethanamine
(C) N-Methyl ethanamine
(D) N-Ethyl – N-methylethanamine
Answer:
(B) N,N-Diethylmethanamine
Explanation:
![]()
properties, uses, identification of primary, secondary and tertiary amines.
OR
Answer:
(D) N-Ethyl – N-methylethanamine
Explanation:
By reaction of methyl amine with two moles of ethyl chloride, N-Ethyl – N-methylethanamine is formed.
![]()
MCQ On Amines Class 12 Chapter 13 Question 3.
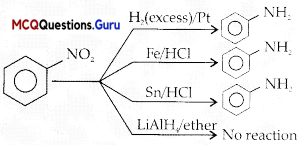
Which of the following reagents would not be a good choice for reducing an aryl nitro compound to an amine?
(A) H2(excess)/Pt
(B) LiAIH4 in ether
(C) Fe and HCI
(D)SnandHCl
Answer:
(B) LiAIH4 in ether
Explanation:
MCQ On Amines Class 12 Chapter 13 Question 4.
The correct increasing order of basic strength for the following compounds is ………..
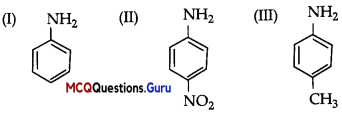
(A) II < III< I
(B) III < I < II
(C) III < II< I
(Ð) III < I < I
Answer:
(Ð) III < I < I
Explanation:
Electron withdrawing group decreases the basic strength while electron releasing groups increases the basic strength of aniline.
Amines Class 12 MCQ Pdf Chapter 13 Question 5.
The best reagent for converting2-Phenylpr0Pam1de into 2-phenyipropananhirie as …………
(A) excess H2
(B) Br2 in aqueous NaOH
(C) Iodine in the presence of red phosphorus
(D) LIAIH4 in ether
Answer:
(D) LIAIH4 in ether
Explanation:
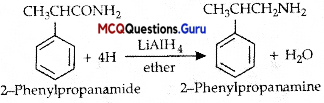
MCQ Of Amines Class 12 Chapter 13 Question 6.
Hinsberg’s reagent which is used to test amines is
(A) Benzene suiphonamide
(B) Benzene diazonium chloride
(C) Benzene sulphonyl chloride
(D) Acetanilide
Answer:
(C) Benzene sulphonyl chloride
Explanation:
Hinsberg’s reagent which is used to test amines is benzene suiphonyl chloride.
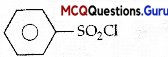
![]()
MCQs On Amines Class 12 Chapter 13 Question 7.
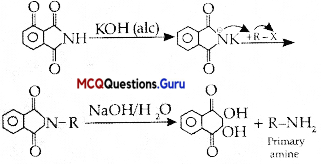
The best method for preparing primary amines from alkyl halides without changing the number of carbon atoms in the chain is:
(A) Hoffmann Bromamide reaction
(B) Gabriel phthalimide synthesis
(C) Sandmeyer reaction
(D) Reaction with NH3 CRI
Answer:
(B) Gabriel phthalimide synthesis
explanation:
Gabriel phthalimide synthesis is used to get primary amines from alkyl halides without changing the number of carbon atoms.
Amines MCQs Class 12 Chapter 13 Question 8.
Write IUPAC name of the following compound:
![]()
(A) N,N-Dimethylpropanamifle
(B) I ,l-Dimethylbutanamifle
(C) N-Methylpentan-1-amifle
(D) N,N.Dimethylbutafl-1-amine
Answer:
(D) N,N.Dimethylbutafl-1-amine
Explanation:
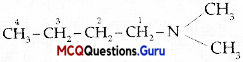
IUPAC name: N, N – Dimethyl butan-1-aniine
Amines MCQ Pdf Class 12 Chapter 13 Question 9.
Thecorrect IUPAC name for CH2=CHCH2NHCH3 is:
(A) Aflylmethylamine
(B) 2-ariiino-4-pentene .
(C) 4-aminopent-1-ene
(D) N-methylprop-2-en-1-amifle
Answer:
(D) N-methylprop-2-en-1-amifle
Explanation:
CH2 = C2HC1H2NIICH3
IUPAC naine: N-methylprop-2-en-1-amifle
Class 12 Chemistry Chapter 13 MCQ Question 10.
Which of the following is a 3° amine?
(A) 1-methylcydohexylamine
(B) Triethylaniine
(C) tert-butylamirie
(D) N-methylaniline
Answer:
(B) Triethylaniine
Explanation:
Triethylamine [(CHN] is a 30 or tertiary amine as nitrogen atom contains three ethyl groups
Amines MCQ Questions Class 12 Chapter 13 Question 11.
Methylamine reacts with HNO2 to form
(A) CH3-O-N=O
(B) CH3-O-CH3
(C) CH3OH
(D) CH3CHO
OR
The gas evolved when methylamine reacts with nitrous acid is:
(A) NH3
(B) N2
(C) H2
(D) C2H6
Answer:
(C) H2
Explanation:
Methylamine reacts with HNO2 to form CH3OH
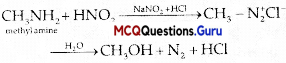
Answer:
(B) N2
Explanation:
Nitrogen gas is evolved.
![]()
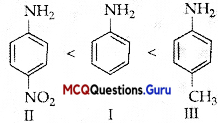
Class 12 Chemistry Amines MCQ Chapter 13 Question 12.
Arrange the following in increasing order of basic strength:
Aniline, p-nitroaniline and p-toludine.
(A) Aniline < p-Nitroaniline < p-Toluidine
(B) Aniline < p-Toluidine < p-Nitroaniline
(C) p-Toluidine < p-Nitroaniline < Aniline
(D) p-Nitroaniline < Aniline < p-Toluidine
Answer:
(D) p-Nitroaniline < Aniline < p-Toluidine
Explanation:
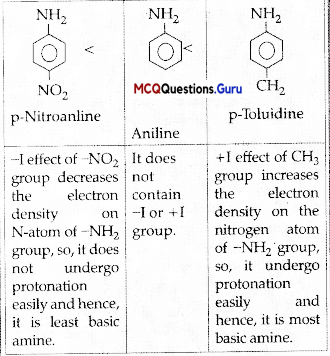
MCQ On Aromatic Amines Class 12 Chapter 13 Question 13.
Which of the following species are involved in the carbylamine test ?
(A) R-NC
(B) COCl2
(C) NaNO2 + HCl
(D) All of the above
Answer:
(A) R-NC
Explanation:
In the carbylamine test, a primary amine reacts with chloroform and KOH to form alkyl isocyanide (i.e. R-NC) having unpleasant smell.
Question 14.
Which of the following amines can be prepared by Gabriel synthesis ?
(A) Isobutyl amine
(B) Toluene
(C) N-methylbenzylamine
(D) Aniline
Answer:
(A) Isobutyl amine
Explanation:
Gabriel phthalamide synthesis cannot be used for preparation of aromatic amines, as aromatic halides do not undergo nucleophilic substitution by salt formed by phthalamide
Question 15.
Which of the following should be most volatile ?
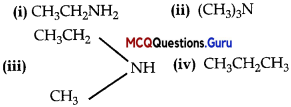
(A) (ii)
(B) (iv)
(C) (i)
(D) (iii)
Answer:
(B) (iv)
Explanation:
Primary and secondary amines form hydrogen bonds and hence are less volatile than corresponding alkanes.
Assertion And Reason Based MCQs
Directions: In the following questions, A statement of Assertion (A) is followed by a statement of Reason (R). Mark the correct choice as.
(A) Both A and R are true and R is the correct explanation of A
(B) Both A and R are true but R is NOT the correct explanation of A
(C) A is true but R is false
(D) A is false and R is True
Question 1.
Assertion (A): Acylation of amines gives a monosubstituted product whereas alkylation of amines gives poly substituted product.
Reason (R): Acyl group sterically hinders the approach of further acyl groups.
Answer:
(C) A is true but R is false
Explanation:
In alleviation, an amine can react with alkyl halide to form next higher class of amine caused by the presence of electron pair on nitrogen which makes amine to behave as nucleophile and alkyl halide thus undergo nucleophilic substitution reaction. When primary and secondary amines react with acid chlorides, anhydrides and esters to give monosubstituted amides as products. Acylation is carried out in the presence of a base stronger than the amine like pyridine which causes the shift of the equilibrium to the right side.
![]()
Question 2.
Assertion (A): Acetanilide is less basic than aniline.
Reason (R): Acetylation of aniline results in decrease of electron density on nitrogen.
Answer:
(A) Both A and R are true and R is the correct explanation of A
Explanation:
Acetanilide is less basic than aniline as in amides the carbonyl group (C=0) is a stronger dipole than N-C dipole. Therefore, the ability of N-C group to act as H-bond acceptor (as a base) is restricted in the presence of a C=0 dipole.
Question 3.
Assertion (A): N, N-Diethylbenzene sulphonamide is insoluble in alkali.
Reason (R): Suiphonyl group attached to nitrogen atom is strong electron withdrawing group.
Answer:
(A) Both A and R are true and R is the correct explanation of A
Explanation:
N,N-Diethylbenzenesulphonamide is insoluble in alkali because it has no acidic hydrogen. Sulphonyl group attached to nitrogen atom is electron withdrawing group
Question 4.
Assertion (A): Aromatic 1° amines can be prepared by Gabriel Phthalimide synthesis.
Reason (R): Aryl halides do not undergo nucleophilic substitution with anion formed by phthalimide.
Answer:
(D) A is false and R is True
Explanation:
Aromatic 1° amines cannot be prepared by Gabriel Phthalimide synthesis because aryl halides do not undergo nucleophilic substitution with anion formed by phthalimide.
Case-Based MCQs
I. Read the passage given below and answer the following questions:
Greater is the stability of the substituted ammonium cation, stronger should be the corresponding amine as a base. Thus, the order of basicity of aliphatic amines should be: primary > secondary > tertiary, which is opposite to the inductive effect based order. Secondly, when the alkyl group is small, like -CH3 group, there is no steric hindrance to H-bonding. In case the alkyl group is bigger than CH3 group, there will be steric hinderance to H-bonding. Therefore, the change of nature of the alkyl group, e.g., from -CH3 to -C2H5 results in change of the order of basic strength.
In the following questions a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
(A) Assertion and reason both are correct statements and reason is correct explanation for assertion.
(B) Assertion and reason both are correct statements but reason is not correct explanation for assertion.
(C) Assertion is correct statement but reason is wrong statement.
(D) Assertion is wrong statement but reason is correct statement.
Question 1.
Assertion (A): (CH3)2NH > CH3NH2 > (CH3)3N > NH3 is the order of basic strength in case of methyl substituted amines.
Reason (R): The inductive effect, solvation effect and steric hindrance of the alkyl group decides the basic strength of alkyl amines in the aqueous state.
Answer:
(A) Assertion and reason both are correct statements and reason is correct explanation for assertion.
Explanation:
(CH3)2NH > CH3NH2 > (CH3)3N > NH3 is the order of basic strength in case of methyl substituted amines as the inductive effect, solvation effect and steric hinderance of the alkyl group decides the basic strength of alkyl amines in the aqueous state.
Question 2.
Assertion (A): (C2H5)2NH > (C2H5)3N > C2H5NH2 > NH3 is the order of basic strength in case of ethyl substituted amines.
Reason (R): The change of nature of the alkyl group, does not result in change of the order of basic strength.
Answer:
(C) Assertion is correct statement but reason is wrong statement.
Explanation:
(C2H5)2NH > (C2H5)3N > C2H5NH2 > NH3 is the order of basic strength in case of ethyl substituted amines. The change of nature of the alkyl group, results in change of the order of basic strength.
![]()
Question 3.
Assertion (A): Greater is the stability of the substituted ammonium cation, stronger is the corresponding amine as a base.
Reason (R): The order of basicity of aliphatic amines is: primary > secondary > tertiary.
Answer:
(C) Assertion is correct statement but reason is wrong statement.
Explanation:
Greater is the stability of the substituted ammonium cation, stronger is the corresponding amine as a base but the inductive effect, solvation effect and steric hinderance of the alkyl group decides the basic strength of alkyl amines in the aqueous state.
Question 4.
Assertion (A): Amines behave as a Lewis base.
Reason (R): Amines have an unshared pair of electrons on nitrogen atom.
Answer:
(A) Assertion and reason both are correct statements and reason is correct explanation for assertion.
Explanation:
Amines behave as a Lewis base as they have an unshared pair of eleolrons on nitrogen atom.
OR
Assertion (A): Solubility of amines in water decreases with increase in molar mass.
Reason (R): Intermolecular H bonds formed by the higher amines are weaker.
Answer:
(C) Assertion is correct statement but reason is wrong statement.
Explanation:
Lower aliphatic amines are soluble in water because thcv can form hydrogen bonds with water molecules. 1 lowever, solubility decreases with increase in molar mass of amines due to increase in size of the hydrophobic alkvl part.
II. Read the passage given below and answer the following questions: (1 x 4 = 4)
Benzene ring in aniline is highly activated. This is due to the sharing of lone pair of nitrogen with the ring which results in increase in the electron density on the ring and hence facilitates the electrophilic attack. The substitution mainly takes place at ortho and para positions because electron density is more at ortho and para positions. On reaction with aqueous bromine all the ortho and para positions get substituted resulting in the formation of 2,4,6-tribromoaniline. To get a monobromo compound, the amino group is acetylated before bromination. After bromination, the bromoacetanilide is acid hydrolysed to give the desired halogenated amine.
In the following questions, a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices :
(A) Assertion and reason both are correct statements and reason is correct explanation for assertion.
(B) Assertion and reason both are correct statements but reason is not correct explanation for assertion.
(C) Assertion is correct statement but reason is wrong statement.
(D) Assertion is wrong statement but reason is correct statement.
Question 1.
Assertion (A): Benzene ring is aniline is highly deactivated.
Reason (R): In aniline, the sharing of lone pair of nitrogen with the ring increases the electron density on the ring.
Answer:
(D) Assertion is wrong statement but reason is correct statement.
Explanation:
Benzene ring in aniline is highly activated.
Question 2.
Assertion (A): In aniline -NH2 group facilitates the electrophilic attack.
Reason (R): It is due to decrease in electron density on the ring.
Answer:
(C) Assertion is correct statement but reason is wrong statement.
Explanation:
In aniline, -NHL group facilitates the electrophilic attack because the sharing of, lone pair of nitrogen with the ring increases the electron density on the ring.
![]()
Question 3.
Assertion (A): In aniline, the substitution mainly takes place at ortho and para positions.
Reason (R): The electron density is more at ortho and para positions.
Answer:
(C) Assertion is correct statement but reason is wrong statement.
Explanation:
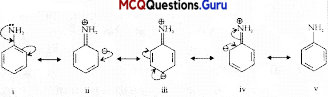
In aniline, the electron density is more at ortho and para positions than meta position, so, the substitution mainly takes place at ortho and para positions. The above resonating structures of aniline show more electron density at the ortho and para positions.
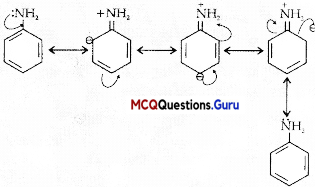
Question 4.
Assertion (A): The amino group of aniline is acetylated before bromination.
Reason (R): It is due to the strong deactivating effect of -NH2 group.
Answer:
(C) Assertion is correct statement but reason is wrong statement.
Explanation:
-NHL group of aniline is acetylated before bromination due to the strong activating effect of -NIL group.
III. Read the passage given below and answer the following questions:
The main problem encountered during electrophilic substitution reactions of aromatic amines is that of their very high reactivity. Substitution tends to occur at ortho and para- positions. If we have to prepare monosubstituted aniline derivative, how can the activating affect of -NH2 group be controlled? This can be done by protecting the -NH2 group By acetylation with acetic anhydride, then carrying out the desired substitution followed by hydrolysis of the substituted amide to the substituted amine.
Question 1.
Give the major product of the following reaction:
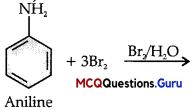
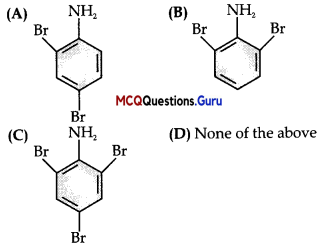
Answer:
(C)
Explanation:
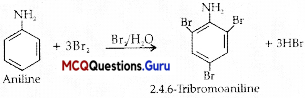
Question 2.
What is the major product A of the following reaction:
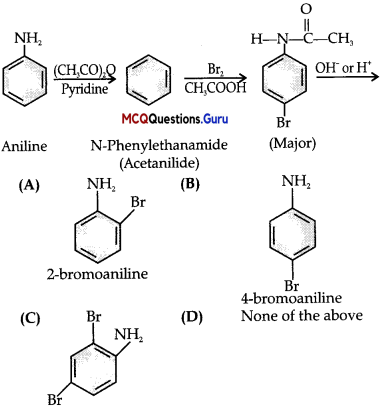
Answer:
(B)
Explanation:
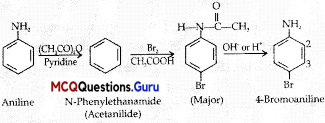
Question 3.
Why is the activating effect of -NHCOCH3 group in the above reaction less than the activating effect of amino group?
(A) Due to mesomeric effect of benzene ring.
(B) Due to inductive effect of alkyl group.
(C) Due to resonance effect of acetanilide.
(D) All of the above.
Answer:
(C) Due to resonance effect of acetanilide.
Explanation:
The lone pair of electrons on nitrogen of acetanilide interacts with oxygen atom due to resonance as shown below:

Hence, the lone pair of electrons on nitrogen is less available for donation to benzene ring by resonance. Therefore, activating effect of – NHCOCH3 group is less than that of amino group.
![]()
Question 4.
Aniline is a resonance hybrid of
(A) 3 structures
(B) 6 structures
(C) 2 structures
(D) 5 structures
Answer:
Option (D) is correct.
Explanation:
Aniline is a resonance hybrid of 5 structures.