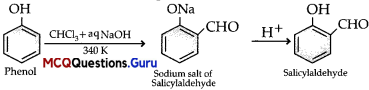
Haloalkanes and Haloarenes Class 12 MCQs Questions with Answers
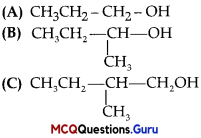
Haloalkanes And Haloarenes Class 12 MCQ Chapter 10 Question 1.
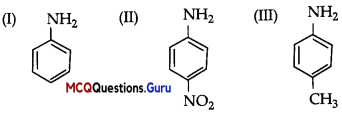
The order of reactivity of following alcohols with halogen acids is:
Answer:
Option (B) is correct.
Explanation:
The reactivity order of alcohols towards halogen acids is 3° >2° > 1° as the stability of carbocations is of the order 3°>2°>1°.
Haloalkanes And Haloarenes MCQ Chapter 10 Question 2.
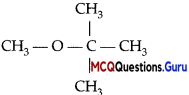
Which of the following alcohols will yield the corresponding alkyl chloride on reaction with concentrated HCl at room temperature?
Answer:
Option (D) is correct.
Explanation:
As tertiary carbocation is more i stable, so tertiary alcohols will yield the corresponding alkyl chloride on reaction with : concentrated HCl at room temperature. While primary and secondary alcohols require the presence of a catalyst ZnCl2.

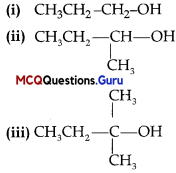
MCQ Of Haloalkanes And Haloarenes Class 12 Question 3.
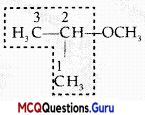
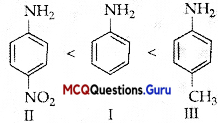
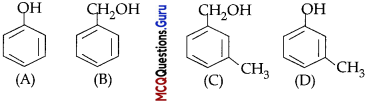
Arrange the following compounds in increasing order of their boiling points:
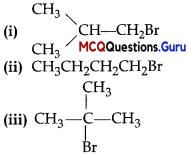
(A) (ii) < (i) < (iii)
(B) (1) < (ii) < (iii)
(C) (iii) < (i) <(ii)
(D) (iii) < (ii) < (i)
Answer:
(C) (iii) < (i) <(ii)
Explanation:
Boiling points of isomeric haloalkanes decrease with increase in branching as with increase in branching surface area decreases which leads to decrease in intermolecular forces.
Haloalkane And Haloarene MCQ Chapter 10 Question 4.
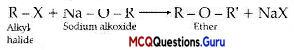
The conversion of an alkyl halide into an alcohol by aqueous NaOH is classified as
(A) a dehydrohalogenation reaction
(B) a substitution reaction
(C) an addition reaction
(D) a dehydration reaction
Answer:
(B) a substitution reaction
Explanation:
R – X + NaoH →R – OH + NaX
MCQ On Haloalkanes And Haloarenes Class 12 Chapter 10 Question 5.
Which of the following alkyl halides will undergo reaction most readily?
(A) (CH3)3C-F
(B) (CH3)3C-Cl
(C) (CH3)3C-Br
(D) (CH3)3C-I
Answer:
(D) (CH3)3C-I
Explanation:
(CH3)3C-I will undergo \(S_{N}^{1}\) reaction most readily as C-I bond is weakest, due to the large difference in the size of carbon and iodine.

Class 12 Haloalkanes And Haloarenes MCQ Chapter 10 Question 6.
Which reagent will you use for the following reaction?
CH3CH2CH2CH3 → CH3CH2CH2CH2Cl + CH3CH2CHClCH3
(A) Cl2/UV light
(B) NaCl + H2SO4
(C) Cl2 gas in dark
(D) Cl2 gas in the presence of iron in dark
Answer:
(A) Cl2/UV light
Explanation:
The given reaction is a free radical substitution reaction. It occurs in presence of ultraviolet light or at high temperature or peroxides which are free radical generators. Free radical substitution cannot take place in dark.
Class 12 Chemistry Haloalkanes And Haloarenes MCQ Question 7.
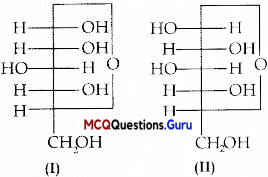
In which of the following molecules carbon atom marked with asterisk (*) is asymmetric?
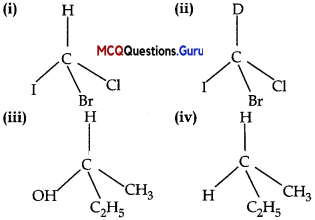
(A) (i), (ii), (iii), (iv)
(B) (i), (ii), (iii)
(C) (ii), (iii), (iv)
(D) (i), (iii), (iv)
Answer:
(B) (i), (ii), (iii)
Explanation:
Asvmmetric’Chiral carbon atom is that in which all of its four valencies lie with four different groups or atoms. In molecules (i), (ii) and (iii), all have asymmetric carbon as each carbon has satisfied all four valencies with four different groups or atoms. In molecule (iv) carbon satisfies two of its valencies with two h\drogen atoms i.c. similar atom. So, it is not an esymmetrn carbon atom.
Haloalkanes And Haloarenes MCQ Class 12 Chapter 10 Question 8.
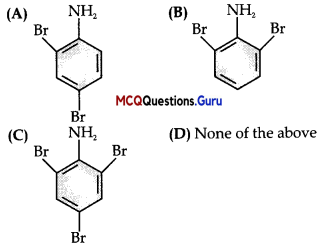
Which of the following structures is enantiomeric with the molecule (a) given below?
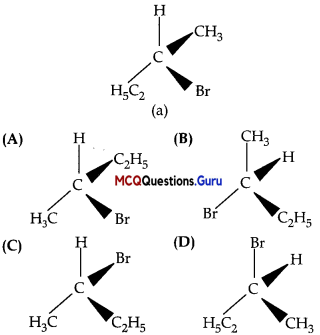
Answer:
Option (A) is correct.
Explanation:
Compound (a) is enantiomer of compound (A) because the configuration of two groups, that is, CH3 and C2H5 in them is reversed at chiral carbon.

Haloalkanes And Haloarenes Class 12 MCQ With Answers Question 9.
Identify the compound Y in the following reaction.
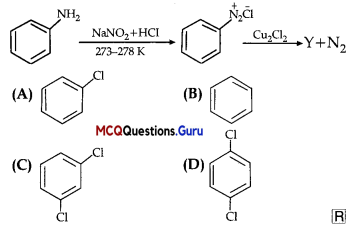
Answer:
Option (A) is correct.
Explanation:
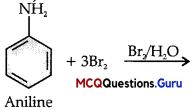
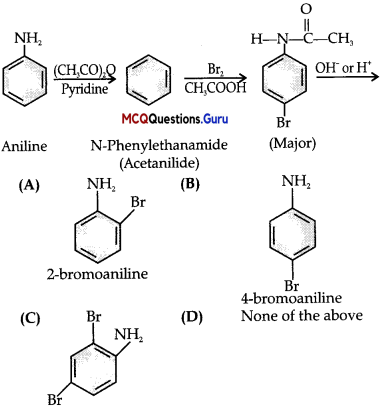
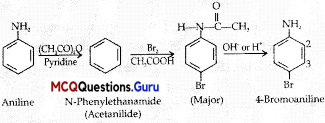
When a primary aromatic .inline is dissolved or suspended in cold aqueous mineral acid and treated with sodium nitrite, ? a diazonium salt is formed. When this freshly prepared diazonium salt is mixed with cuprous chloride, diazonium group is replaced by Cl. hen, chlorobenzene is formed which is Y in this reaction.
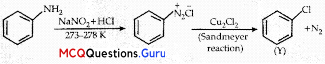
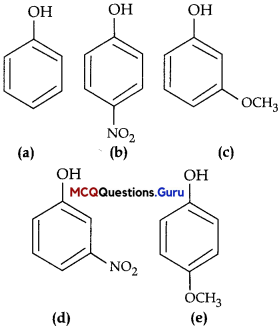
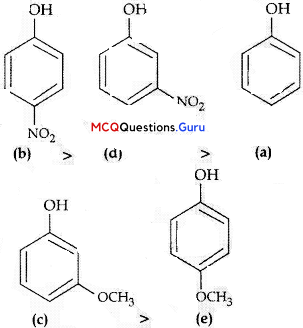
Haloalkanes And Haloarenes Class 12 MCQs Chapter 10 Question 10.
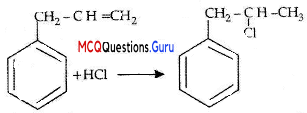
What is A in the following reaction?
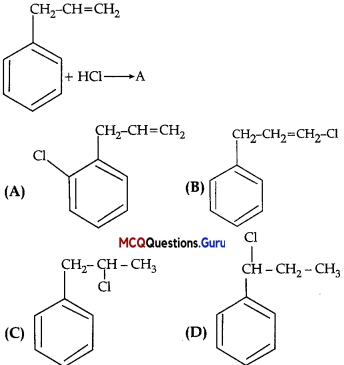
Answer:
Option (C) is correct.
Explaiuition:
In this reaction, addition of HCl takes 1 place on doubly bonded carbons in accordance with Markovnikov’s rule, that is, addition of negative addendum will take place on that carbon which has lesser number of hydrogen.
Haloarenes And Haloalkanes MCQ Chapter 10 Question 11.
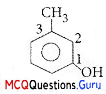
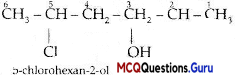
The IUPAC name of the compound shown below is:
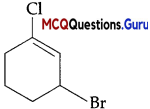
(A) 2-bromo-6-chlorocyclohex-l-ene
(B) 6 -bromo-2-chlorocyclohexene
(C) 3-bromo-1-chlorocyclohexene
(D) l-bromo-3-chlorocyclohexene
Answer:
(C) 3-bromo-l-chlorocyclohexene

Explanation:
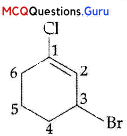
IUPAC name: 3-bromo-l-chlorocyclohexene
MCQs Of Haloalkanes And Haloarenes Chapter 10 Question 12.
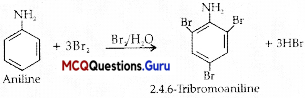
Toluene reacts with a halogen in the presence of iron (III) chloride giving ortho and para halo compounds. The reaction is.
(A) Electrophilic elimination reaction
(B) Electrophilic substitution reaction
(C) Free radical addition reaction
(D) Nucleophilic substitution reaction
Answer:
(B) Electrophilic substitution reaction
Explanation:
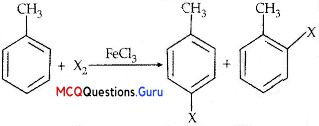
Class 12 Chemistry Chapter 10 Haloalkanes And Haloarenes MCQ Question 13.
Which of the following is halogen exchange reaction?
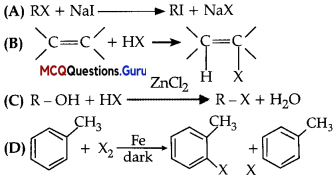
Answer:
Option (A) is correct.
Explanation:
Halogen exchange reactions are those in which one halide replace-another This reaction is known as Finkelstein reaction.
Chemistry Haloalkanes And Haloarenes MCQ Chapter 10 Question 14.
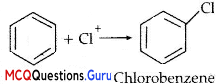
Chlorobenzene is formed by reaction of chlorine with benzene in the presence of AlCl3. Which of the following species attacks the benzene ring in this reaction?
(A) Cl–
(B) Cl+
(C) AlCl3
(D) [AlCl4 ] –
Answer:
(B) Cl+
Explanation:
Cl– is an electrophile formed by the following reaction:
AlCl3 + Cl2 → [A1C13] + Cl+
Cl+ attacks the benzene ring to give chlorobenzene.


Chemistry Class 12 Haloalkanes And Haloarenes MCQ Chapter 10 Question 15.
Reaction of C6H5CH2Br with aqueous sodium hydroxide follows:
(A) \(S_{N}^{1}\) mechanism
(B) \(S_{N}^{2}\) mechanism
(C) Any of the above two depending upon the temperature of reaction
(D) Saytzeff rule
Answer:
(A) \(S_{N}^{1}\) mechanism
Explanation:
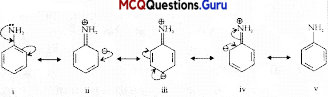
C6H5CH2 will follow mechanism on reaction with aqueous sodium hydroxide since the carbocalion formed C6H5CH2is a resonance stabilized cation Benzylic halides show high reactivity towards the \(S_{N}^{1}\) reaction. The carbocation thus formed gets stabilized through resonance as shown in the structure.
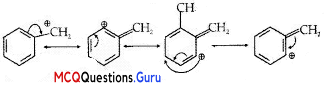
MCQ Questions Of Haloalkanes And Haloarenes Chapter 10 Question 16.
Arrange the following compounds in the increasing order of their
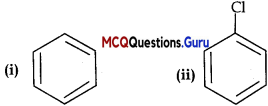
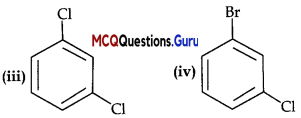
(A) (i) <(ii) < (iii) < (iv)
(B) (i) < (iii) < (iv) < (ii)
(C) (iv) < (iii) < (ii) < (i)
(D) (ii) < (iv) < (iii) < (i)
Answer:
(A) (i) <(ii) < (iii) < (iv)
Explanation:
Density increases with increase in molecular mass.
Assertion And Reason Based MCQs
Directions: In the following questions, A statement of Assertion (A) is followed by a statement of
Reason (R): Mark the correct choice as
(A) Both A and R are true and R is the correct explanation of A
(B) Both A and R are true but R is NOT the correct explanation of A
(C) A is true but R is false
(D) A is false and R is True
MCQs On Haloalkanes And Haloarenes Chapter 10 Question 1.
Assertion (A): Aryl halides undergo nucleophilic substitution reactions with ease.
Reason(R): The carbon halogen bond in aryl halides has partial double bond character.
Answer:
(D) A is false and R is True
Explanation:
Aryl halides are less reactive towards nucleophilic substitution reactions because of the carbon halogen bond in aryl halides has partial double bond character.
MCQ Questions On Haloalkanes And Haloarenes Chapter 10 Question 2.
Assertion (A): Hydrolysis of (-) -2-bromooctane proceeds with inversion of configuration.
Reason (R): This reaction proceeds through the formation of a carbocation.
Answer:
(D) A is false and R is True
Erplanation.
Hydrolysis of (-)-2-bromooctane proceeds through the formation of a caibocation following \(S_{N} 1\) reaction.

Ch 10 Chemistry Class 12 MCQ Question 3.
Assertion (A): tert-Butyl bromide undergoes Wurtz reaction to give 2, 2,3, 3-tetramethylbutane.
Reason (R): In Wurtz reaction, alkyl halides react with sodium in dry ether to give hydrocarbon containing double the number of carbon atoms present in the halide.
Answer:
(A) Both A and R are true and R is the correct explanation of A
Explanation:
In Wurtz reaction, alkyl halides react with sodium In dry ether to give hydrocarbon containing double the number of carbon atoms present in the halide so tert-Butyl bromide undergoes Wurtz reaction to give 2, 2,3, 3-tetramethylbutane.
MCQ Of Haloalkanes And Haloarenes Class 12 Chapter 10 Question 4.
Assertion: Phosphorus chlorides (tri and penta) are preferred over thionyl chloride for the preparation of alkyl chlorides from alcohols.
Reason: Phosphorus chlorides give pure alkyl halides.
Answer:
(B) Both A and R are true but R is NOT the correct explanation of A
Explanation:
Thionyl chloride is best halogen carrier to convert alcohol to alkyl halide because it gives by-products in gaseous slate. Thus, we get pure alkyl halide in this reaction.
Question 5.
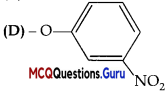
Assertion (A): Presence of a nitro group at ortho or para position increases the reactivity of haloarenes towards nucleophilic substitution.
Reason (R): Nitro group, being an electron withdrawing group decreases the electron density over the benzene ring.
Answer:
(A) Both A and R are true and R is the correct explanation of A
Explanation:
Nitro group being an electron withdrawing group, decreases the electron density of benzene ring thus increasing the reactivity of haloarenes towards nucleophilic substitution.
Question 6.
Assertion (A): In monohaloarenes, further electrophilic substitution occurs at ortho and para positions.
Reason (R): Halogen atom is a ring deactivator.
Answer:
(B) Both A and R are true but R is NOT the correct explanation of A
Explanation:
Halogens are ortho-para directing due to (+ M) or (+R) effect. Moreover, they are deactivating due to high electronegativity.

Question 7.
Assertion (A): It is difficult to replace chlorine by -OH in chlorobenzene in comparison to that in chloroethane.
Reason (R): Carbon-chlorine (C-Cl) bond in chlorobenzene has a partial double bond character due to resonance.
Answer:
(A) Both A and R are true and R is the correct explanation of A
Explanation:
Chlorobenzene is very less reactive to nucleophilic substitution reaction by -OH group as Carbon-chlorine (C-Cl) bond in chlorobenzene has a partial double bond character due to resonance.
Question 8.
Assertion (A): Aryl iodides can be prepared by reaction of arenes with iodine in the presence of an oxidising agent.
Reason (R): Oxidising agent oxidises I2 into HI.
Answer:
(C) A is true but R is false
Explanation:
Oxidising agent like (HIO3) converts HI to I2 otherwise HI may reduce aryi halide to arenes.
5HI + HIO3 → 3H3O + 3I2.
Case-Based MCQs
I. Read the passage given below and answer the following questions:
Nucleophilic substitution reaction of haloalkane can be conducted according to both \(S_{N} 2\) and \(S_{N} 2\) mechanisms. However, which mechanism it is based on is related to such factors as the structure of haloalkane, and properties of leaving group, nucleophilic reagent and solvent.
Influences of halogen:
No matter which mechanism the nucleophilic substitution reaction is based on, the leaving group always leave the central carbon atom with electron pair. This is just the opposite of the situation that nucleophilic reagent attacks .the central carbon atom with electron pair.

Therefore, the weaker the alkalinity of leaving group is , the more stable the anion formed is and it will be more easier for the leaving group to leave the central carbon atom; that is to say, the reactant is more easier to be substituted. The alkalinity order of halogen ion is I– < Br– < Cl– < F– and the order of their leaving tendency should be I– > Br– > Cl– > Therefore, in four halides with the same alkyl and different halogens, the order of substitution reaction rate is RI > RBr > RC1 > RF.
In addition, if the leaving group is very easy to leave, many carbocation intermediates are generated in the reaction and the reaction is based on SN! mechanism. If the leaving group is not easy to leave, the reaction is based on \(S_{N} 2\) mechanism.
Influences of solvent polarity:
In \(S_{N} 1\) reaction, the polarity of the system increases from the reactant to the transition state, because polar solvent has a greater stabilizing effect on the transition state than the reactant, thereby reduce activation energy and accelerate the reaction. In \(S_{N} 2\) reaction, the polarity of the system generally does not change from the reactant to the transition state and only charge dispersion occurs. At this time, polar solvent has a great stabilizing effect on Nu than the transition state, thereby increasing activation energy and slow down the reaction rate.
For example, the decomposition rate (\(S_{N} 1\)) of tertiary chlorobutane in 25° water (dielectric constant 79) is 300000 times faster than in ethanol (dielectric constant 24). The reaction rate (\(S_{N} 2\)) of 2-bromopropane and NaOH in ethanol containing 40% water is twice slower than in absolute ethanol. In a word, the level of solvent polarity has influence on both \(S_{N} 2\) and \(S_{N} 2\) reactions, but with different results. Generally
speaking, weak polar solvent is favorable for \(S_{N} 2\) reaction, while strong polar solvent is favorable for \(S_{N} 1\) reaction, because only under the action of polar solvent can halogenated hydrocarbon dissociate into carbocation and halogen ion and solvents with a strong polarity is favorable for solvation of carbocation, increasing its stability. Generally speaking, the substitution reaction of tertiary haloalkane is based on \(S_{N} 2\) mechanism in solvents with a strong polarity (for example, ethanol containing water).
The following questions are multiple choice questions. Choose the most appropriate answer:
Question 1.
mechanism is favoured in which of the following solvents:
(A) benzene
(B) carbon tetrachloride
(C) acetic acid
(D) carbon disulphide
Answer:
(C) acetic acid
Question 2.
Nucleophilic substitution will be fastest in case of:
(A) 1-Chloro-2,2-dimethyl propane
(B) 1 -Iodo-2,2-dimethyl propane
(C) 1-Bromo-2,2-dimethyl propane
(D) 1 -Fluoro-2,2-dimethyl propane
Answer:
Option (B) is correct.

Question 3.
\(S_{N} 1\) reaction will be fastest in which of the following solvents?
(A) Acetone (dielectric constant 21)
(B) Ethanol (dielectric constant 24)
(C) Methanol (dielectric constant 32)
(D) Chloroform (dielectric constant 5)
Answer:
(C) Methanol (dielectric constant 32)
Question 4.
Polar solvents make the reaction faster as they:
(A) destabilize transition state and decrease the activation energy
(B) destabilize transition state and increase the activation energy
(C) stabilize transition state and increase the activation energy
(D) stabilize transition state and decrease the activation energy
Answer:
(C) stabilize transition state and increase the activation energy
Question 5.
\(S_{N} 1\) reaction will be fastest in case of:
(A) l-Chloro-2-methyl propane
(B) l-Iodo-2-methyl propane
(C) 1-Chlorobutane
(D) 1-Iodobutane
Answer:
(B) l-Iodo-2-methyl propane
II. Read the passage given below and answer the following questions:
Alkyl/Aryl halides may be classified as mono, di or polyhalogen compounds depending on one, two or more halogen atoms in their structures. Alkyl halides are prepared by free radical halogenation of alkanes, addition of halogen acids to alkenes and replacement of -OH group of alcohols with halogens using phosphorus halides, thionyl chloride or halogen acids. Aryl halides are prepared by electrophilic substitution to arenes. The following questions are multiple choice questions. Choose the most appropriate answer:
Question 1.
Complete the reaction:
H3C – Br + AgF →
(A) H3C-Br + AgF → H3C-F + AgBr
(B) H3C-Br + AgF → Br-CH2F + AgH
(C) H3C-Br + AgF → [Ag(CH3)]F + Br
(D) None of the above
Answer:
(A) H3C-Br + AgF → H3C-F + AgBr
Explanation:
H,C-Br + AgF → H3C-F + AgBr.

Question 2.
Name the major monohalo product of the following reaction:
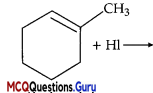
(A) 1-Iodo-l-methyl cyclohexane
(B) 1-Iodomethyl cyclohexane
(C) 1-Chloro cyclohexane
(D) None of the above
Answer:
(B) 1-Iodomethyl cyclohexane
Explanation:
According to Markovnikov’s rule, iodine will add to the carbon atom having less number of hydrogen atoms.
Question 3.
2-Bromopentane, 2-Bromo-2-methylbutane, 1-Bromopentane Write the compound which is most reactive towards (3-elimination reaction.
(A) 2-Bromopentane
(B) 1- Bromopentane
(C) 2-Bromo-2-methylbutane
(D) None of the above
Answer:
(C) 2-Bromo-2-methylbutane
Explanation:
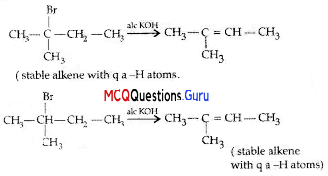
Question 4.
Which of the following is halogen exchange reaction?
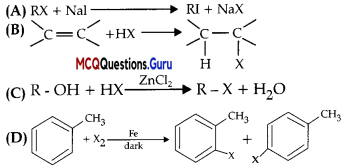
Answer:
Option (A) is correct.
Explanation:
RX + Nal → RI + NaX
It is halogen exchange reaction as in this reaction both R and Na exchanges halogens.

OR
Arrange the following compounds in increasing order of their boiling points.
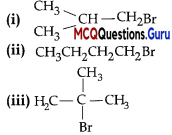
(A) (ii) < (iii) < (i)
(B) (i) < (ii) < (iii)
(C) (iii) < (i) < (ii)
(D) (iii) < (ii) < (i)
Answer:
(C) (iii) < (i) < (ii)
Explanation:
Boiling point of (i) is 364 K, boiling point of (ii) is 375 K. boiling point of (iii) is 346 K As the branching increases in the isomeric alkyl halides, the boiling point decreases.
III. Read the passage given below and answer the following questions:
The objects which are non-superimposable on their mirror image (like a pair of hands) are said to be chiral and this property is known as chirality. Chiral molecules are optically active, while the objects, which are, superimposable on their mirror images are called achiral. These molecules are optically inactive. The above test of molecular chirality can be applied to organic molecules by constructing models and its mirror images or by drawing three dimensional structures and attempting to superimpose them in our minds. There are other aids, however, that can assist us in recognising chiral molecules. One such aid is the presence of a single asymmetric carbon atom.
In these questions a statement of assertion followed by a statement of reason is given . Choose the correct answer out of the following choices.
(A) Assertion and Reason both are correct statements and Reason is correct explanation for Assertion.
(B) Assertion and Reason both are correct statements but Reason is not correct explanation for Assertion.
(C) Assertion is correct statement but reason is wrong statement.
(D) Assertion is wrong statement but Reason is correct statement.
Question 1.
Assertion (A): The stereoisomers related to each other as non-superimposable mirror images are called enantiomers
Reason (R): Enantiomers possess identical physical properties.
Answer:
(B) Assertion and Reason both are correct statements but Reason is not correct explanation for Assertion.t.
Explanation:
The stereoisomers related to each other as non-superimposable mirror images are called enantiomers and they possess identical physical properties.

Question 2.
Assertion (A): A racemic mixture containing two enantiomers in equal proportions will have zero optical rotation.
Reason (R): This is because the rotation due to one isomer will be cancelled by the rotation due to the other isomer.
Answer:
(A) Assertion and Reason both are correct statements and Reason is correct explanation for Assertion.
Explanation:
A racemic mixture is an equimolar mixture of d and 1 forms. It is optically inactive due to externa] compensation as rotation of one l form is cancelled bv other form.
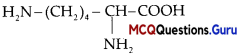
Question 3.
Assertion (A): Butan-2-ol is a chiral molecule.
Reason (R): It has 4 different groups attached to carbon atom.
Answer:
(A) Assertion and Reason both are correct statements and Reason is correct explanation for Assertion.
Explanation:
Hutan-2-ol is a chiral molecule as it has 4 different functional groups attached to the tetrahedral carbon atom.
Question 4.
Assertion (A): Propan-2-ol is an achiral molecule.
Reason (R): Carbon is called asymmetric carbon or stereocentre.
Answer:
(C) Assertion is correct statement but reason is wrong statement.
Explanation:
Propan-2-ol is an achiral molecule as it does not contain an asymmetric carbon, as all the four groups attached to the tetrahedral carbon are not different
IV. Read the passage given below and answer the following questions:
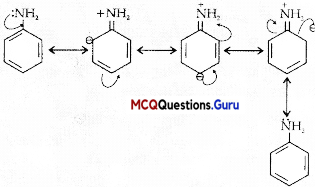
Aryl halides are extremely less reactive towards nucleophilic substitution reactions due to the following reasons:
(i) In haloarenes, the electron pairs on halogen atom are in conjugation with ir-electrons of the ring.
(ii) In haloalkane, the carbon atom attached to halogen is sp3 hybridised while in case of haloarene, the carbon atom attached to halogen is sp2 -hybridised.
(iii) In case of haloarenes, the phenyl cation formed as a result of self-ionisation will not be stabilised by resonance.
The following questions are Multiple Choice Questions. Choose the most appropriate answer:
Question 1.
A primary alkyl halide would prefer to undergo
(A) \(S_{N} 1\) reaction
(B) \(S_{N} 2\) reaction
(C) a-Elimination
(D) Racemisation
Answer:
(B) \(S_{N} 2\) reaction
Explanation:
A primary alkyl halide would prefer to undergo \(S_{N} 2\) reaction.

Question 2.
Which of the following alkyl halides will undergoes \(S_{N} 1\) reaction most readily?
(A) (CH3)3C-F
(B) (CH3)3C-Cl
(C) (CH3)3C-Br
(D) (CH3)3C-I
Answer:
(D) (CH3)3C-I
Explanation:
(CH)3C-I being a tertiary alkyl halide will most readily undergo \(S_{N} 1\) reaction.
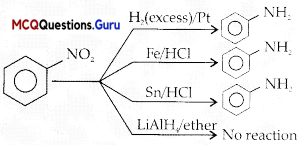
Question 3.
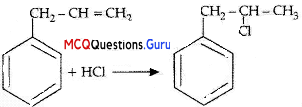
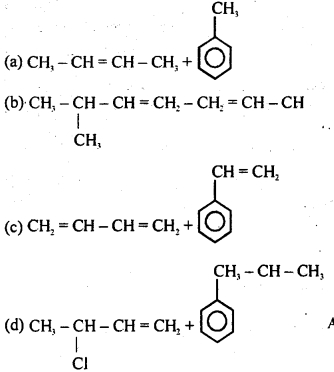
What is ‘A’ in the following reaction?
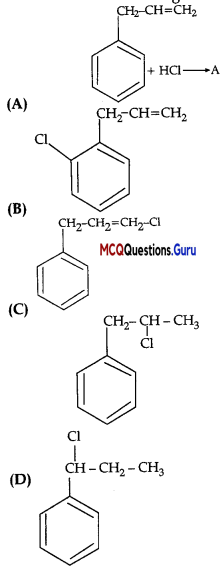
Answer:
Option (D) is correct.
Explanation:
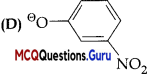
Question 4.
Reaction of C6H5CH2Br with aqueous sodium hydroxide follows .
(A) \(S_{N} 1\) mechanism
(B) \(S_{N} 2\) mechanism
(C) Any of the above two depending upon the temperature of reaction
(D) Saytzeff rule.
Answer:
(D) Saytzeff rule.
Explanation:
C6H5-CH2 is stable cation so favours the progress of reaction by \(S_{N} 1\) mechanism
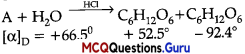
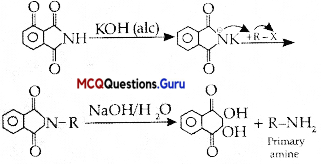
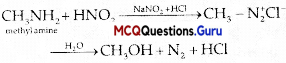
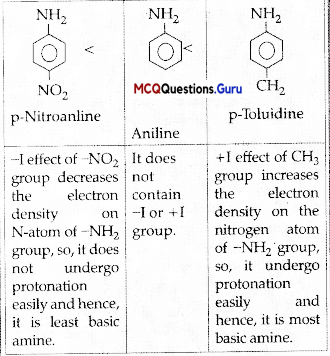
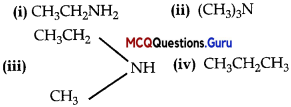

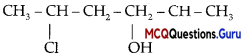

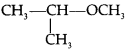 is ………….
is ………….