NCERT Solutions for Class 11 Chemistry Chapter 10 s-Block Elements (Alkali and Alkaline Earth Metals)
These Solutions are part of NCERT Solutions for Class 11 Chemistry. Here we have given NCERT Solutions for Class 11 Chemistry Chapter 10 The s-Block Elements (Alkali and Alkaline Earth Metals).
Question 1.
What are the common physical and chemical features of alkali metals ?
Answer:
| Property | Li | Na | K | Rb | Cs | Fr |
| Atomic number | 3 | 11 | 19 | 37 | 55 | 87 |
| Atomic mass (g mol-1) | 6-94 | 22-99 | 39-10 | 85-47 | 132-91 | (223) |
| Atomic (Metallic) radius (pm) | 152 | 186 | 227 | 248 | 265 | — |
| Ionic radius (pm) | 76 | 102 | 138 | 152 | 167 | — |
| Ionization enthalpy (ΔiHi) in kJ mol-1 | 520 | 496 | 419 | 403 | 376 | — |
| Ionisation enthalpy (Δi-Hj) in kJ mol-1 |
7298 | 4562 | 3051 | 2633 | 2230 | — |
| Hydration enthalpy (Δ HHydration ) in kJ mol |
-506 | -406 | -330 | -310 | -276 | — |
| Electronegativity (Pauling scale) | 10 | 0-9 | 0-8 | 0-8 | 0-7 | — |
| Density/g cm-3 (at 293 K) | 0-53 | 0-97 | 0-86 | 1-53 | 1-90 | — |
| Melting point/K | 453-5 | 370-8 | 336-2 | 312-0 | 301-5 | — |
| Boiling point/K E° (V) at 298 K for | 1620 | 1154-4 | 1038-5 | 961-0 | 978-0 | — |
| M+ (aq) + e–—— > M (s) | -3-03 | -2-71 | -2-93 | -2-93 | -2-92 | — |
Now let us try to account for the general trends in the physical properties with the help of the available data.
1. Atomic radii. Alkali metals have maximum value of the atomic radii (metallic radii) in their respective periods and these values further increase down the group. Although the last element in each period e., the noble gas element has more value of the atomic radius, but it is van der Waals radius and not atomic or metallic radius as in alkali metals. Therefore, it is not proper to make a comparison in their values.
Explanation. We know that the atomic radius is the distance of the outermost electron present in the valence shell of an atom from the center of its nucleus. Being the first element in the respective periods, alkali metal atoms have maximum atomic .radii since their atoms have only one electron in the valence shell. As a result, the magnitude of the force of attraction with the nucleus is the minimum. Down the group, the atomic radius increases mainly because of the gradual increase in the number of electron shells.
In addition to this, the magnitude of the screening effect also increases which decreases the attraction between the valence 5-electron with the nucleus of the atom. No doubt, the nuclear charge also increases which is likely to increase the attraction of the electrons with the nucleus resulting in the decreased atomic size. However, its magnitude is very small compared to the screening effect. The net result is what we actually observe i.e., atomic sizes of the elements increase down the group.
2. Ionic radii. Alkali metal atoms form monovalent cations by the loss of valence s (ns1) The cationic radius is less as compared to that of the atom. As the values indicate, the radii increase down the group.
Explanation. A monovalent cation is formed by the loss of the only electron present in the valence shell of the atom. As a result, the remaining electrons are pulled closer to the nucleus of the atom causing a decrease in the cationic size. We all know that the size of the ion (cation or anion) is linked to that the corresponding atom. Since the atomic radii increase down the group; so are the ionic radii.
3. Ionisation enthalpies. The ionisation enthalpy as all of us know, is the minimum amount of energy needed to remove the most loosly bound electron from a neutral isolated atom in the gaseous state. Its unit is kJ mol-1. It can also be expressed as ionisation potential with electron volt (eV) per atom as the unit
(1 eV per atom = 96-472 kJ mol ).
Alkali metals have the lowest ionisation enthalpies in their respective periods and these further decrease down the group. The first (ΔiH1) and second (ΔiH2) ionisation energies .
Explanation. The low ionisation enthalpies of the alkali metals may be attributed to very large atomic sizes as a result of which the valence x-electron (ns1) can be readily removed. These values decrease down the group because of decrease in the magnitude of the force of attraction with the nucleus on account of increased atomic radii and magnitude of screening effect. However, there is a large difference in ΔiH1 and ΔiH2 values of a particular element. For example, A,H] value of sodium is 496 kJ mol-1 while its Δi H2 value is 4562 kJ mol1. It is mainly because of the reason that with the release of the first electron, the monovalent cation (M+) is highly symmetrical and has nearest noble gas configuration. Therefore, the loss of second electron is very difficult comparatively. The difference in the Δi H1 and Δi H2 values can be, thus, justified.
4. Electronegativity of an element is the relative electron attracting tendency of its atom for a shared pair in a bond. Alkali metals have low electronegativities which means that their electron attracting tendencies are low. These values tend to decrease down the group.
Explanation. As the alkali metal atoms have ns1 electronic configuration, this means that they have electron releasing rather than electron accepting tendency. Thus, they have low values of electronegativities as is evident from the data listed in the Table 10.2. Since the atomic sizes increase down the group, there is gradual decrease in capacity of the atoms to hold their valence electrons. Therefore, the electronegativities decrease down the group.
5. Oxidation states and electropositive character. All the members of the alkali metal family are strongly electropositive (tendency to form positive ion) and show + 1 oxidation states in their compounds. The electropositive character further increases down the group.
Explanation. Alkali metal atoms have strong urge to lose the valence electron and to form monovalent cation due to their low ionisation energies. These are therefore, highly electropositive in nature. This character i.e., electron releasing tendency increases further down the group as the ionisation energy decreases.
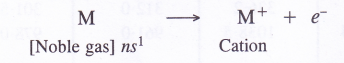
6. Metallic character. Group 1 elements are typical metals and are so soft that most of them can be easily cut with a knife. The metallic character further increases down the group.
Explanation. The metallic character of the element is related to the electron releasing tendency of its atom to form positive ion. The strength of the metallic bond depends upon magnitude of the attractive forces present in the kernels and valence electrons in the electron sea. Smaller the size of the kernel and more is the number of valence electrons, stronger will be the metallic bond and greater will be the hardness of the metal. In alkali metals, the kernels are of large size (it depends upon the atomic size) and there is only one valence electron. Therefore, metallic bond is rather weak and as a result, alkali metals are quite soft. They can be cut even with a knife. Lithium, the first element is the hardest since the kernel is the smallest in size.
7. Melting and boiling points. Alkali metals have low melting and boiling points which further decrease down the group.
Explanation. As the atoms belonging to the alkali metals have large size, their binding energies in the crystal lattice are quite low. As a result, they have low melting points. These decrease down the group because of the increase in atomic size. The explanation for the trends in boiling points is also similar.
8. Density . Alkali metals are very light. The first three members of the family are even lighter than water. The density increases downwards.
Explanation. Since the alkali metal atoms have large size, these are not so closely packed in space and have, therefore, low densities. The densities are expected to decrease further down the group because the atomic sizes increase. But the trend is the reverse which means that the densities increase downwards as listed in Table 10.2. This is because of the reason that the atomic masses of the elements increase and the effect of increase in atomic mass is more compared to increase in atomic size. Therefore, the density (mass/volume) increases downwards. However, there is an exception i.e., the density of potassium is less than that of sodium. This may be probably due to the reason that there is an abnormal increase in atomic size and atomic volume as we move from sodium to potassium.
9. Hydration enthalpies. Hydration enthalpy is the energy which is released when the ion of a particular element takes up molecules of water and gets hydrated. In general, smaller the size of the ion, more is its tendency to get hydrated or more is the hydration enthalpy. In the alkali metals, the hydration enthalpies of the monovalent cations (M+) are low due to their large size. These further decrease down the group since the ionic size increases.
Explanation : The hydration involves attraction between the ion and the surrounding molecules of water. Thus, smaller the size of the ion, more will be the magnitude of the charge on it and more will be its capacity to hydrate. Among the alkali metals, Li+ ion has the maximum hydration enthalpy. Therefore, the salts of lithium are mostly hydrated in nature e.g., LiCl.2H2O.
10. Colouration to the flame. The members of the alkali metals, particularly the chlorides of these metals when heated on a platinum wire impart characteristic colours to the flame of the burner. For example,

Explanation. As the alkali metals have low ionisation enthalpies, the energy of the flame causes the excitation of the electrons to higher energy state away from the nucleus. When these excited electrons jump back to the normal or ground states, the emitted radiations fall in the visible region of light. Therefore, the alkali metals impart colour to the flame. Among these metals, lithium has the minimum size and requires maximum energy for the electronic excitation.
Therefore, for the same excitation energy available (from the temperature of the flame) the energy level to which the electron in lithium atom jumps will be lower as compared to sodium. This also means that when the electron returns to the ground state, the energy released or the frequency of the radiations emitted will be minimum (E = hv). In other words, the frequencies of the radiations emitted will follow the order Li < Na < K < Rb < Cs. This is responsible for the colours of flame imparted by different metal atoms as their salts.
11. Photoelectric effect. Alkali metals (except Li) exhibit photoelectric effect. It may be defined as the phenomenon of the ejection of electrons from the surface of a metal when electrons of certain frequency strike against the metal surface. In nut-shell, the electrons are emitted from the metal surface under the influence of the striking photons.
Explanation. The cause of the photoelectric effect is the low ionisation enthalpies of the alkali metals. The striking photons of light have sufficient energy to knock out the electrons from the metal surface. Since lithium has comparatively high ionisation enthalpy due to its very small atomic size, the striking photons do not have sufficient energy to overcome the force of attraction of the electrons with the nucleus. The element, therefore, does not exhibit photoelectric effect in the visible region of light where the energy of striking photons is not sufficient to cause photoelectric effect.
Chemical Properties of alkali metals:
Alkali metals are highly reactive chemically because of their low ionisation enthalpies and enthalpy of atomisation. Some of the important chemical properties of the members of the family are discussed.
1. Reaction with air. When freshly cut, alkali metals have luster but their surfaces get tarnished when exposed to air due to the formation of a layer of oxide, hydroxide and carbonate.
4M + O2 → 2MzO
M2O + H2O → 2MOH
2MOH + CO2 → M2CO3 + H2O
The alkali metals can not be placed in air. Similarly, these are also not placed in water due to strong affinity. These are normally kept in chemically inert solvents such as kerosene.
2. Reaction with oxygen. Alkali metals combine with oxygen upon heating to form different oxides depending upon their nature. Lithium forms a normal oxide (Li2O), sodium forms peroxide (Na2O2) while potassium and rest of the metals form superoxides (MO2 where M = K, Rb and Cs) upon heating in oxygen.

Reactivity with oxygen increases from Li to Cs
Explanation. The stabilities of these oxides are linked with the relative sizes of the cations and anions involved and also upon the charges present on them. In general :
A smaller cation can stabilise a smaller anion while a larger cation can stabilise a larger anion.
The size of the Li+ ion is the smallest and has a strong positive field around it. It can combine only with a small anion such as oxide ion (O2-) with a strong negative field around it. Na+ ion due to bigger size has a weaker positive field around it and, therefore, can stabilise peroxide ion (O22-) which has also a weaker negative field around it. Thus, sodium forms peroxide (Na2O2). The remaining cations (K+, Rb+ and Cs+) are still bigger in size and the magnitude of the positive field around them decreases in the same order. They can stabilise only an anion with a very weak negative field around it i.e., superoxide ion (Of).
The valence bond structures of oxide, peroxide and superoxide ions are given below :
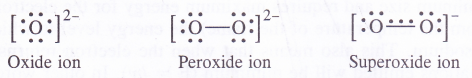
The superoxide ion has a three electron bond and due to the presence of unpaired electron, it is paramagnetic in nature. On the other hand, both oxide and peroxide ions have no unpaired electrons. These are, therefore, diamagnetic in nature. The magnetic nature of these ions can also be explained on the basis of molecular orbital theory. For details, consult chemical bonding and molecular structure
3. Reaction with water (Formation of hydroxides). Alkali metals, their oxides, peroxides and even superoxides dissolve in water to form hydroxides which are soluble and are called alkalies (water soluble hydroxides are known as alkalies). For example,

The reactions of metals with water are so highly exothermic that the hydrogen gas evolved catches fire accompanied by explosion. Therefore, alkali metals are not kept in contact with water. These are kept under kerosene.
Question 2.
Discuss the general characteristics and gradation in properties of alkaline earth metals.
Answer:
The important physical properties of the members of the family are listed in the Table
| Property | Be | Mg | Ca | Sr | Ba | Ra (Radioactive) |
| Atomic number | 4 | 12 | 20 | 38 | 56 | 88 |
| Atomic mass | 901 | 2 4-31 |
40-08 | 87-62 | 137-33 | 226-03 |
| Atomic radius/pm (Metallic) | 112 | 160 | 197 | 215 | 222 | |
| Ionic radius/pm | 31 | 72 | 100 | 118 | 135 | 148 |
| Ionisation enthalpy (ΔiH1) | 899 | 737 | 590 | 549 | 503 | 509 |
| (kJ mof1)(ΔiH2) | 1757 | 1450 | 1146 | 1064 | 965 | 979 |
| Hydration enthalpy | ||||||
| in (kJ mof1) | -2494 | -1921 | -1577 | -1443 | -1305 | |
| Electronegativity | ||||||
| (Pauling scale) | 1-5 | 1-2 | 1-00 | 1-0 | 0-9 | 0-9 |
| Density/g mol-1 (at 293 K) | 1-85 | 1-74 | 1-55 | 2-63 | 3-62 | 5-5 |
| Melting point/K | 1560 | 924 | 1424 | 1062 | 1002 | 973 |
| Boiling point/K, | 2745 | 1363 | 1767 | 1655 | 2078 | 1973 |
| E° (Y) at 298 K for | ||||||
| M2+ (aq) + 2e– A—> M(s) | -1-97 | -2-36 | -2-84 | -2-89 | -2-92 | -2-92 |
Based upon the above data, let us discuss the trends in the important physical properties of these elements.
1. Atomic radii. The atomic radii of the alkaline earth metals are fairly large but less than the corresponding alkali metals present in the same period. They increase down the group.
Explanation. The atoms of these elements have only two valence electrons and the magnitude of the force of attraction with the nucleus is quite small. Therefore, these elements have sufficient large atomic sizes as well atomic radii. They tend to increase down the group because of gradual increase in the number of electron shells and magnitude of screening or shielding effect.
2. Ionic radii. All the elements form divalent cation (M2+) by losing the valence electrons. Therefore, the ionic radius is less than the radius of the corresponding atom. These increase down the group as these are linked to the atomic radii of the elements.
3. Ionisation enthalpies. The members of the alkaline earth family have, in general, low ionisation enthalpies due to fairly large atomic sizes. As the atomic sizes increase down the group, the ionisation enthalpies are expected to decrease in the same manner. This is quite evident from the data listed in the Table
The ionisation enthalpies of radium (both AjHj and AjHf) are slightly higher than those of barium while these are expected to be less. This is probably due to the reason that it comes after the lanthanoid series off-block elements which undergo lanthanoid contraction. Therefore, radium undergoes some decrease in atomic size resulting in an increase of ionisation enthalpies.
A comparison of the ionisation enthalpies of the elements present in group 1 and group 2 belonging to the same period indicates that the ΔiH1, values of group 2 elements are higher while the ΔiH2 values are lower. The Δi H [ and Δi H2 both Na and Mg are given to support it.

Explanation. The (A,-Hi) value of Mg is higher than that of Na because of smaller size and more symmetrical electronic configuration. But after losing one electron, Na+ ion acquires the configuration of noble gas neon (1 ,v22.r2//’) while Mg+ ion has still one electron in the valence shell (li22522/>63s1). Due to greater symmetrical electronic configuration, ΔiH2 value of sodium is higher than that of magnesium while its A,-Hi value is less.
4. Hydration enthalpies. Just like alkali metals, the divalent cations of the alkaline earth metals have also tendency to get hydrated. The negative hydration enthalpies are more due to the smaller size of cations as compared to the cations of the alkali metals present in the same period. For example, Li+ (AhydH = – 506 kJ mol 1) ; Be2+ (AhydH = – 2494 kJ mol ). The hydration enthalpies decrease down the group since the catonic size increases
Be2+ > Mg2+ > Ca2+ > Sr2+ > Ba2+
The salts of alkaline earth metals generally exist as hydrated salts. For example, MgCl2 exists as MgGl2. 6H2O while CaCl2 as CaCl2.6H2O. The corresponding salts of alkali metals NaCl and KCl are anhydrous since their cations are not hydrated so extensively.
5.electronegativity. The electronegativity values of alkaline earth metals are quite close to those of alkali metals, though slightly more. These values decrease from beryllium to radium indicating increased tendency to form ionic compounds. The high electronegativity value of beryllium (1-5) indicates that the element tends to form covalent compounds.
6. Oxidation states and electropositive character. All the members of the family exhibit + 2 oxidation states in their compounds and form divalent cations (M2+). This appears to be rather unacceptable because the enthalpy needed to form monovalent cation (M+) in all these elements is less than what is required to form a divalent cation (M2+). But ionisation enthalpy alone is not the sole criteria for the formation of the cations. A number of other factors are also involved. These are briefly discussed as follows :
- The divalent cation has the configuration of the nearest noble gas element while the monovalent cation is yet to achieve it.
- In the solid state, divalent cation results in a stronger lattice than monovalent cation because of its smaller ionic radius and greater magnitude of positive charge as compared to monovalent cation. A stronger lattice releases more lattice enthalpy in its formation than a weaker lattice.
- In aqueous solution, the divalent cation has a greater tendency to get hydrated because of its smaller size and stronger positive field as compared to monovalent cation. Thus, the hydration enthalpy which is released is more in case of divalent cation as compared to monovalent cation.
- The tendency to get hydrated is maximum in Be2+ ions due to their smallest size and is least in Ba2+ Their ionic mobilities follow the reverse order i. e., Be2+ < Mg2+ < Ca2+ < Sr2+ < Ba2+.
used upon the above discussion, one can conclude that divalent cations are formed in preference of monovalent cations when alkaline earth metals participate in the formation of chemical compounds. This can be further supported by considering the enthalpy changes which occur in the formation of MgCl and MgCl2 in aqueous solution.
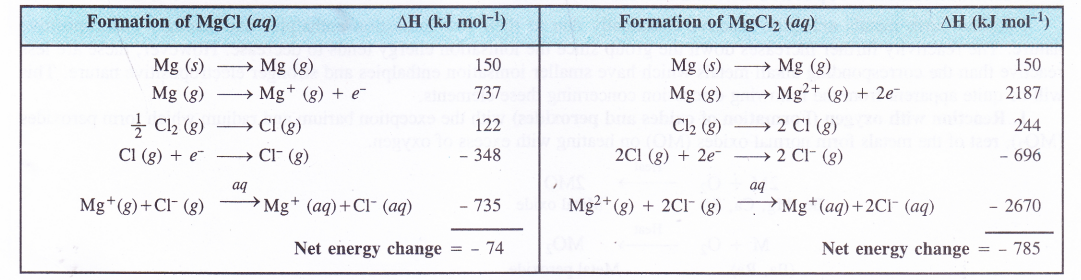
Thus, in aqueous solution, MgCl2 is formed in perference to MgCl. The alkaline earth metals are electropositive in nature as they form positive (divalent) cations and this character increases down the group. However, the first element beryllium (Be) is the least electropositive due to its very small size and high ionisation energy. The components of beryllium are mostly covalent in nature.
7. Metallic character. Alkaline earth metals have stronger metallic bonds as compared to the alkali metals present in the same period. For example, magnesium is harder and denser than sodium.
Explanation. The stronger metallic bonds in alkaline earth metals as compared to alkali metals may be attributed to the smaller kernel size and more number of valence electrons (two) compared to alkali metals (there is only one valence electron). The stronger bonding also results in more close packed arrangement.
8. Density. Alkaline earth methls are denser than the alkali metals present in the same period because these are more closely packed due to smaller size and.stronger metallic bonds. However, the trend in the density is not uniform. It initially increases from Be to Ca and then decreases from Ca to Ba. The irregular trends in densities are due to the difference in the crystal structures of these metals.
9. Melting and boiling points. The melting and boiling points of these metals are higher than those of the alkali metals present in the same period. This is quite expected also since they have more packed crystal lattice as compared to the alkali metals. But the data as given in the Table 10.4 indicates an irregular trend in these values. This is because of the reason that the atoms adopt different crystal structures in these metals.
- Chemical Properties of Alkaline Earth Metals
Alkaline earth metals are quite reactive chemically due to their low ionisation enthalpies and strongly electropositive nature. The reactivity further increases down the group since the ionisation energy tends to decrease. However, these are less reactive than the corresponding alkali metals which have smaller ionisation enthalpies and stronger electropositive nature. This will be quite apparent from the following discussion concerning these elements.
1. Reaction with oxygen (Formation of oxides and peroxides) with the exception barium and radium which form peroxides (MO2), rest of the metals form normal oxides (MO) on heating with excess of oxygen.
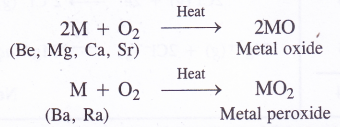
Beryllium is quite unreactive and reacts only above 600°C when the powder form of the metal is heated with oxygen. Magnesium burns brightly on heating in oxygen and the reactivity with oxygen increases down the group. The enthalpies of formation of these oxides are quite high and these are quite stable in nature.
2. Reaction with water (Formation of hydroxides). The members of alkaline earth metals are less reactive towards water as compared to the corresponding alkali metals because these drfe’less electropositive in nature. Beryllium, the first member of the family has no action while magnesium reacts with boiling water or steam to form magnesium hydroxide (also called milk of magnesia). The rest of the members react even with cold water and their reactivity increases down the group

The hydroxides are also formed, when the metal oxides are dissolved in water BeO
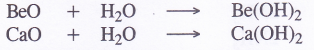
3. Reaction with hydrogen (Formation of hydrides). The members of the family, except beryllium, combine with hydrogen directly upon heating to form metal hydrides

The hydride of beryllium (BeH2) can be prepared indirectly by the reduction of BeCl2 with LiΔiH4 dissolved in anhydrous ether
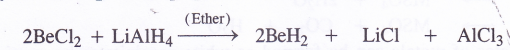
BeH2 and MgH2 are covalent in nature while the hydrides of the other metals have ionic structure. The ionic hydrides such as CaH2 (also called hydrolith) react with water to evolve hydrogen gas

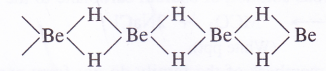
Structure of BeH2. It exists as a polymeric solid (BeH2)„ in which each H atom is involved in a three centred bond and exists as a bridge
4. Reaction with halogens (Formation of halides). The members of the family combine directly with halogens at appropriate temperatures to form corresponding halides i. e., MX2. The halides can also be formed by the action of halogen acid (HX) on oxides, hydroxides and carbonates of these metals.

Berlyllium chloride can be obtained by passing vapour of chorine over BeO and carbon at 1073 K
![]()
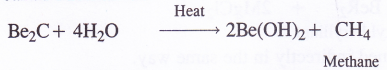
5. Reaction with carbon (Formation of carbides). Beryllium carbide is formed by heating BeO with coke (C) at about 1900 – 2000°C temperature when a brick red coloured ionic carbide Be2C is formed. It reacts with water to evolve methane gas. Be2C is quite often called Methanide.
The other elements of group-2 generally form ionic carbides with the formula MC2 (M = Mg, Ca, Sr and Ba) by heating the metal directly with carbon (coke) in an electric arc furnace. These can also be formed by heating the metal oxides with coke.

Out of these carbides, calcium carbide evolves acetylene gas on reacting with water. It is therefore, quite often called acetylide.
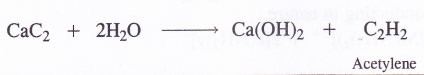
6. Reaction with nitrogen (Formation of nitrides). Upon heating in an atmosphere of nitrogen (N2), the members of alkaline earth family form nitrides with the formula M3N2. For example,
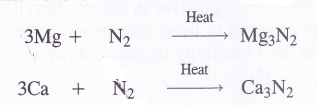
These metal nitrides are crystalline solids. They react with water forming corresponding hydroxides and liberating ammonia gas.
Mg3N2 + 6H2O → 3Mg(OH)2 + 2NH3
Ca3N2+ 6H20 → 3Ca(OH)2 + 2NH3
7. Reaction with sulphuric acid (Formation of sulphates). Sulphates of the members of group 2 elements can be formed by the action of dilute H2SO4
M+ H2SO4 →MSO4 + H2
Alternatively, these can be obtained by reacting dilute H2SO4 with oxides, hydroxides or carbonates of these elements.
MO + H2SO4 → MSO4 + H2O
M(OH)2+ H2SO4 → MSO4 +2H2O
MCO3 +H2SO4 → MSO4 + CO2 + H2O
8. Formation of carbonates. Carbonates of metals can be formed as white precipitates by passing vapours of CO2 in limited amount through aqueous solutions of the metal hydroxides.
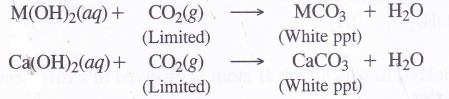
These can also be formed by adding aqueous solution of sodium carbonate to the aqueous salt solution e.g., metal chlorides.
![]()
9. Complex formation. Generally, the members of the family do not form complexes. However, smaller ions like Be2 + and Mg2+ form complexes with the electron donor species. For example, the stable complexes of Be are : [BeF3]–, [BeF4]2- and [Be(H2O)4]2+.Chlorophyll the complex compound of Mg plays an important role in photosynthesis. Ca, Sr and Ba form complexes only with strong complexing agents like acetylacetone and EDTA etc.
10. Formation of organo-metallic compounds. Both beryllium and magnesium form a number of organo-metallic compounds containing M—C bonds with certain organic compounds. For example, magnesium reacts with alkyl halide in the presence of dry ether to give Grignard reagent.

BeCl2 reacts with Grignard reagent to form dialkyl beryllium.

Dialkyls or diaryls of Ca, Sr and Ba can also be formed indirectly in the same way.
11. Reducing nature. Just like alkali metals, the members of the family also act as reducing agents. However, their reducing strengths are comparatively less since their ionisation enthalpies are higher and they have less negative reduction potentials (E°) as compared to the alkali metals. The alkaline earth metals behave as a reducing agents by losing their valence electrons to form divalent cations (M2+). The reducing character increases down the group due to decrease in the magnitude of ionisation enthalpies and also due to increase in the values of negative reduction potentials of the elements.
The first element beryllium (Be) is expected to behave as a very weak reducing agent because of its small atomic radius and high values of ionisation enthalpies (ΔiH1 and ΔiH2). However, it behaves as a reducing agent due to large enthalpy of hydration as well as sublimation (also called enthalpy of atomisation).
12. Solution in liquid ammonium. Like alkali metals, the metals belonging to alkaline earth family (group 2) dissolve in liquid ammonia to give deep blue solution which is conducting in nature.
![]()
Question 3.
Why are alkali metals not found in nature ?
Answer:
Alkali metals are highly reactive due to low ionisation enthalpy and strong electro-positive character. They do not occur in free or native state and are always combined with other elements. As a result, alkali metals are not generally found in nature.
Question 4.
Find the oxidation state of sodium in Na2O2
Answer:
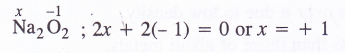
Question 5.
Explain why is sodium less reactive than potassium.
Answer:
This is mainly due to higher ionisation enthalpy (ΔiH1 = 496 kJ mol-1) of sodium as compared to potassium (A,H- = 419 kJ mol-1). As a result, the potassium is more electropositive and stronger reducing agent than sodium. It also reacts with water more violently than sodium. Thus, sodium is less reactive than potassium.
Question 6.
Compare the alkali metals and alkaline earth metals with respect to
(1) ionisation enthalpy
(2) basicity of oxides
(3)solubility of hydroxides.
Answer:
(1) Ionisation enthalpy. The ionisation enthalpy of alkaline earth metals (group 2) is more as compared to alkali metals (group 1) present in the same period due to smaller size and more symmetrical configuration. For example,
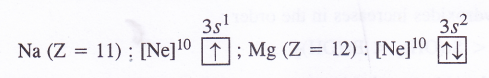
(2) Basicity of oxides. Oxides of alkali metals are stronger bases as compared to those of alkaline earth metals present in the same period. This is quite evident from the fact that when Na2O is dissolved in water, NaOH formed is a stronger base than when MgO is dissolved in water to form Mg(OH)2.
Na2O + H2O → 2NaOH ; MgO + H2O→ Mg(OH)2
This is on account of lesser ionisation enthalpy of alkhli metals as compared to alkaline earth metals. Therefore, NaOH can release OH’ ions in solution more readily than Mg(OH)2.
(3) Solubility of hydroxides. Alkali metal hydroxides are more soluble in water as compared to the hydroxides of alkaline earth metals present in the same period. This is on account of higher lattice enthalpy of the hydroxides of alkaline earth family as compared to those of alkali metals.
Question 7.
In what ways does lithium show similarity to magnesium in its chemical behaviour ?
Answer:
Similarity in Lithium and Magnesium (Diagonal Relationship)
In the discussion of group-1 elements we have seen that lithium, the first member of the family differs from the rest of the members (congeners) in most of the characteristics. However, it has a close resemblance with magnesium, the second element of alkaline earth metals (group 2) which it is linked diagonally. In other words, lithium and magnesium exhibit diagonal resemblance or diagonal relationships. In fact the first three elements of second period (Li, Be, B) show diagonal similarity with the elements (Mg, Al, Si) of third period placed on the right hand side.
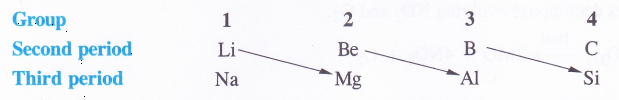
It may be noted that this relationship is noticed only in these pairs of elements and not anywhere else in the periodic table.Cause of Diagonal Relationship. The cause of diagonal relationship is due to opposing trends in the main periodic properties of the elements along a period and down the group. For example, both atomic and ionic radii decrease along a period and increase down the group. Ionisation enthalpy, electron gain enthalpy and electronegativity follow opposing trends i.e., they increase along a period and decrease down the group. On moving diagonally, these opposing trends tend to cancel mutually and as a result, the elements listed above show diagonal relationalship.
Question 8.
Explain why alkali and alkaline earth metals cannot be obtained by chemical reduction.
Answer:
The metals belonging to both these families are very strong reducing agents. It is, therefore not possible to reduce their oxides by reacting with common reducing agents like carbon (coke), zinc etc. These are normally isolated by carrying out the electrolysis of the salts of these metals in the molten state.
Question 9.
Why are potassium and cesium, rather than lithium used in photoelectric cells ?
Answer:
The ionisation enthalpy of lithium is quite high. The photons of light are not in a position to eject electrons from the surface of lithium metal. Therefore, photoelectric effect is not noticed. However, both potassium and cesium have comparatively low ionisation enthalpies. This means that the electrons can quite easily be ejected from the surface of these metals when photons of certain minimum frequency (threshold frequency v°) strike against their surface.
Question 10.
When an alkali metal is dissolved in liquid ammonia, the solution can acquire different colours. Explain the reason for this type of colour change.
Answer:
Solubility in liquid ammonia. Alkali metals dissolve in liquefied ammonia to give highly conducting solution with blue colour.
Explanation. Alkali metals due to their low ionisation energies, ionise in ammonia solution to form ammoniated cations and ammoniated electrons.

The blue colour of the solution is attributed to the fact that when light falls on the ammoniated electrons, they absorb energy corresponding to red colour and the transmitted light has a blue colour. The electrical conductivity of the solution is because of ammoniated cations as well as ammoniated electrons.
- In concentrated solution (3 M solution) the colour changes from blue to bronze which is copper-coloured. The blue solution is paramagnetic while the concentrated solution is diamagnetic in nature.
- The presence of free ammoniated electrons makes the blue solution reducing in nature.
- When dry ammonia gas is passed over heated metals, amides are formed and hydrogen gas is evolved.

Alkali metal amides are powerful reducing agents due to the presence of amide (NH2~) ions.
Question 11.
Beryllium and magnesium do not give colour to the flame while other alkaline earth metals do so. Explain.
Answer:
Colouration to the flame. With the exception of beryllium and magnesium, the rest of the elements impart characteristic colours to the flame. For example,

Explanation. The reason for imparting characteristic colours to the flame when volatile chlorides of these metals are heated on the tip of a platinum wire is the same as given under alkali metals. The heat energy provided by the flame is sufficient to excite the electrons to higher energy states. When these electrons jump back to the normal or ground states, the emitted radiations fall in the visible region of light resulting in characteristic colours. Since elements differ in their ionisation enthalpies, they are excited to different extent and also differ in the energy that is emitted as light radiations. This leads to different flame colours.
Beryllium and magnesium, the first two members of the family fail to impart any colours to the flame because of smaller size and high ionisation enthalpies. The energy of the flame is not sufficient to excite the electrons in these atoms.
Question 12.
Discuss the various reactions which occur in Solvay process.
Answer:
Details of the process : The plant used for the manufacturing process is shown
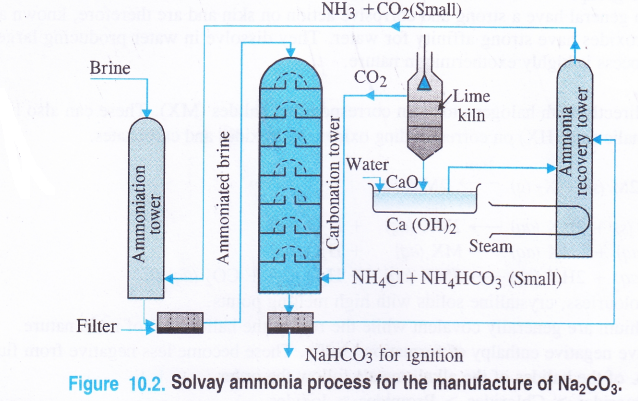
1. Ammoniation Tower. A strong solution of brine (30% NaCl solution) is introduced from the top of the tower made up of iron. A mixture of ammonia and carbon dioxide which are formed in the ammonia recovery tower is led from a side into the tower. As a result, brine gets saturated with ammonia. Soluble impurities of some calcium and magnesium salts like CaCl2 and MgCl2 associated with sodium chloride are precipitated as carbonates by ammonium carbonate which is formed in the reaction.
2NH3 + CO2 + H2O → (NH4)2CO3 CaCl2 + (NH4)2CO3
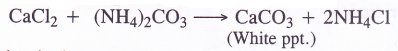
The solution is then passed through filters in order to remove these precipitates.
2. Carbonation Tower. It is also made up of iron and is fitted with a number of horizontal plates. Each plate has a hole in the center covered by a perforated cover. The brine saturated with ammonia (or ammoniated brine) is introduced from the top and the vapours of carbon dioxide from the lime kiln are introduced from the side. As the vapours rise, they come in contact with the ammoniated brine and the following reactions take place.
CO2 + H2O + NH3 →NH4HCO3
NaCl + NH4HCO3 → NaHCO3 + NH4Cl
The temperature in the carbonation tower is between 300 – 310 K (very low) and crystals of sodium hydrogen carbonate are formed. Carbon dioxide needed for the reaction is obtained by heating lime stone (CaCO3) in a lime kiln

Quick lime formed is dissolved in water to form Ca(OH)2 which is led into the ammonia recovery tower as shown in the figure.
3. Filtration. The solution coming out of the cafbonation tower is passed through filters when the precipitated sodium hydrogen carbonate gets separated. The solution containing NH4Cl and small amount of NH4HCO3 is taken to the ammonia recovery tower where it meets calcium hydroxide.
4. Calcination of sodium hydrogen carbonate. Sodium hydrogen carbonate formed above is heated strongly in the absence of air (calcined) in a furnace to give sodium carbonate.
![]()
5. Ammonia recovery tower. In this tower, ammonia is formed by the reaction between NH4Cl and Ca(OH)2 and the reaction mixture is heated with the help of steam coil.
![]()
Ammonium hydrogen carbonate present in the tower also decomposes to evolve NH3 and CO2
![]()
Both these gases are pumped into the ammoniation tower where they take part in the reaction.
Merits of the Ammonia Solvay process. This process is extremely useful because of the following reasons.
- Sodium carbonate (or soda ash) is almost completely pure.
- The process is also very cheap because both NH3 and CO2 are formed in the plant itself.
- There is hardly any pollution problem because the waste like flue gases are not evolved.
Question 13.
Potassium carbonate cannot be prepared by Solvay process. Explain.
Answer:
In Solvay Ammonia process for getting sodium carbonate, NaHCO3 formed gets precipitated and upon heating it gives sodium carbonate. KHCO3 is expected to be formed if the same process is used for the preparation of K2CO3. But as KHCO3 is highly soluble in water, it cannot be isolated.
Question 14.
Lithium carbonate decomposes on heating while sodium carbonate does not.
Answer:
Upon heating, Li2CO3 decomposes to form Li2O and CO2. The smaller size of Li+ ion makes the lattice of Li2O more stable than that of Li2CO3. But due to the bigger size of Na+ ion, the lattice of Na2O is less stable than that of Na2CO3. Therefore, Na2CO3 fails to decompose on heating while lithium carbonate decomposes.
Question 15.
Compare the solubility and thermal stability of the following compounds of alkali metals with those of alkaline earth metals :
(a) Nitrates (b) Carbonates (c) Sulphates
Answer:
(a) Nitrates of alkali and alkaline earth metals.
Solubility. Nitrates of alkali metals are water soluble. Their solubility increases down the group because their lattice ., enthalpy decreases more rapidly than the hydration enthalpy.(Please note that decrease in lattice enthalpy favours solubility of a solid while decrease in hydration enthalpy opposes the same) Nitrates of alkaline earth follow the reverse trends i.e., their solubility decreases down the group because hydration enthalpy decreases more rapidly than the lattice enthalpy.
Thermal stability. The nitrates of alkali metals (except lithium nitrate) upon heating decompose to form corresponding nitrites and evolve oxygen.
![]()
Lithium nitrate decomposes to form lithium oxide and evolves NO2 as well as O2.
![]()
The alkaline earth metal nitrates decompose in the same way as lithium nitrate.
![]()
(b) Carbonates of alkali and alkaline earth metals
Solubility. The trend in the solubility of alkali metal carbonates is the same as that of nitrates i. e., it increases down the group. The trend in the solubility of the alkaline earth metal carbonates is also the same i.e., it decreases down the group
Thermal stability. Except for lithium carbonate?which decomposes upon strong heating to evolve CO2, the carbonates of rest of the alkali metals are quite stable to heat i.e., they donot decompose
![]()
In fact, Li2O is more stable than UCO3 because due to small size of Li+ ion, the lattice of Li2O is quite stable. For rest of the alkali metal carbonates, M2O is less stable than MCO3 due to bigger size of the metal ion. The alkaline earth metal carbonates decompose in the same way as lithium carbonate.
![]()
Due to smaller size of M2+ ion, the lattice of MO is more stable than that of MCO3. However, the stability of metal carbonates increases down the group because of gradual increase in the size of the M2+ ion and lesser stability of MO as compared to MCO3.
(C) Sulphates of alkali and alkaline earth metals
Solubility. The trend in the solubility of both alkali metal sulphates and alkaline earth metal sulphates is the same as in case of nitrates and carbonates.
Thermal stability. Except for Li2SO4 which decomposes upon heating the sulphates of other alkali metals are thermally stable i.e., they do not decompose on heating
![]()
The sulphates of alkaline earth metals also decompose in the same way.
![]()
Question 16.
Starting from sodium chloride, how will you proceed to prepare (1) sodium metal (2) sodium hydroxide (3) sodium peroxide (4) sodium carbonate.
Answer:
(1) Preparation of sodium metal. Sodium metal is formed by carrying out the electrolytic reduction of the salt in the molten state.
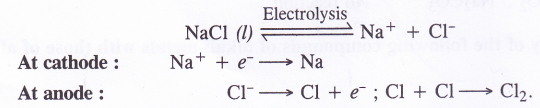
(2) Preparation of sodium hydroxide. Sodium hydroxide is prepared by carrying out the electrolysis of the aqueous solution of sodium chloride either in Nelson’s cell or Castner Kellner cell.
(3) Preparation of sodium peroxide. Sodium chloride is first converted to sodium by electrolytic reduction. The metal is then heated with excess of oxygen at about 573K in an atmosphere free from moisture and carbon dioxide to form sodium peroxide.

(4) Preparation of sodium carbonate. From sodium chloride, sodium carbonate is prepared by Solvay ammonia process.
Anhydrous sodium carbonate is also galled soda ash as it is present in the ashes of certain marine plants. This was the only natural source till 1790. Washing soda, Na2CO3. 10H2O is very popular in laundries for washing clothes. Though a number of methods are available, the most common among them is the Solvay Ammonia Process. This is used for the manufacture of anhydrous sodium carbonate.
Theory : Carbon dioxide is passed through concentrated solution of sodium chloride or brine (about 30%) which is saturated with ammonia to form sodium hydrogen carbonate as follows :

In the presence of Na+ ions present in the solution, sodium hydrogen carbonate gets precipitated. The precipitate is removed by filtration and upon heating, it gives sodium carbonate.
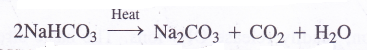
Question 17.
What happens when : –
- Magnesium is burnt in air
- Quick lime is heated with silica
- Chlorine reacts with slaked lime
- Calcium nitrate is heated.
Answer:
(1) A mixture of magnesium oxide and magnesium nitride is formed

(2) Calcium silicate is formed
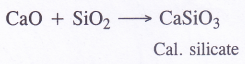
(3) Calcium oxychloride (bleaching powder) is formed

(4) Nitrogen dioxide is evolved

Question 18.
Describe two important uses of each of the following (1) caustic soda (2) sodium carbonate (3) quick lime.
Answer:
1. Uses Sodium hydroxide Or Caustic Soda
- in the manufacture of soap, paper, rayon (artificial silk) and a large number of chemicals,
- in cotton industry for mercerizing (or making unshrinkable) cotton fabrics,
- in the refining of petroleum and also of vegetable oils,
- in the preparation of soda lime (a mixture of NaOH and CaO), ,
- as a cleansing agent for machines and metal sheets.
2. Uses : Sodium carbonate is
- for the manufacture of soap, glass, paper, caustic soda, borax etc.
- as a fusion mixture when mixed with K2CO3.
- for washing of clothes in laundary under the name washing soda,
- in textile industry and also in the refining of petroleum.
- as a laboratory reagent.
3. Uses of Quick Lime :
- It is used for making building materials such as mortar cement etc.
- It is useful in the manufacture of cement, glass, calcium carbide, sodium carbonate etc.
- It is used as- a basic flux in metallurgy.
- It is helpful in tanning industry to remove hairs from hides.
- Quick lime is used for drying a number of gases and alcohol.
Question 19.
Draw the structures of (1) BeCl2 (in solid state) (2) BeCl2 (in vapour state)’.
Answer:
Structure of BeCl2. In solid state, beryllium chloride has a chain structure (polymer) in which the electron pairs from chlorine atoms present on neighbouring molecules are donated to electron deficient Be atom to form co-ordinate bonds as follows :
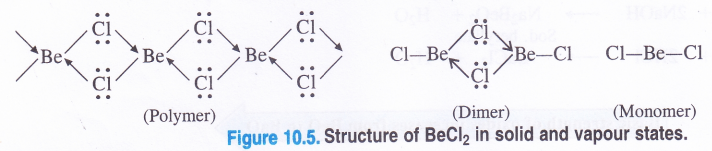
The Be atom in the above chain structure is sp3 hybridised but the Cl—Be—Cl bond angle is considerably less (98°) as compared to normal tetrahedral bond angle of (109-5°). In the vapour state, the compound exists as a dimer (Be atom is sp2 hybridised) which decomposes at about 1000 K to give a monomer in which Be atom is in sp hybridisation state.
Question 20.
The hydroxides and carbonates of sodium and potassium are easily soluble in water while the corresponding compounds of magnesium and calcium are sparingly soluble. Explain.
Answer:
All the compounds are crystalline solids and their solubility in water is guided by both lattice enthalpy and hydration enthalpy. In case of sodium and potassium compounds, the magnitude of lattice enthalpy is quite small as compared to hydration enthalpy since the catonic sizes are large. Therefore, the compounds of sodium and potassium that are mentioned, readily dissolve in water. However, in case of corresponding magnesium and calcium compounds, the cations have smaller sizes and more magnitude of positive charge. This means that their lattice enthalpies are more as compared to the compounds of sodium and potassium. Therefore, the hydroxides and carbonates of these metals are only sparingly soluble in water. For more details, consult text part.
Question 21.
Describe the importance of the following :
(1) Lime stone (2) Cement (3) Plaster of Paris.
Answer:
1. Lime stone or Calcium Carbonate or Marble (CaCOs)
Calcium carbonate occurs in nature as lime stone and also as marble rocks. It can be prepared on small scale by the following methods.
- By passing carbon dioxide through slaked lime in limited amount.
Ca(OH)2 + CO2 → CaCO3 + H2O
Excess of the gas should be avoided as it will lead to soluble calcium bicarbonate.
- By reacting aqueous solutions of sodium carbonate and calcium chloride
Na2CO3 + CaCl2 → CaCO3 + 2NaCl
Properties :
- It is a white fluffy amorphous powder and is only slightly soluble in water.
- Upon heating to 1200 K, it decomposes to evolve carbon dioxide.
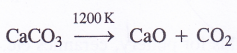
- It liberates carbon dioxide on reacting with dilute mineral acids.
CaCO3 + 2HCl → CaCl2 + H2O + CO2
CaCO3 + H2SO4 → CaSO4 + H2O + CO2
(2) Cement:
Cement is one of the most important materials used in the construction of buildings, dams, bridges, roads etc. In 1824, Joseph Asplin, a mason working in Leeds (U.K.), for the first time, heated a mixture of lime stone, clay and water and allowed the mass to stand for some time when it hardened into a stone like mass. It resembled Portland rock which was an important naturally occurring building stone used in England during those days. He named it portland cement. In India, cement industry came into existence in 1914.
Cement is a greyish fine powder consisting of a mixture of various silicates and aluminates of calcium such as tricalcium silicate (3CaO.SiO2), tricalcium aluminate (3CaO.Al2O3) and dicalcium silicate (2CaO.SiO2). On mixing with water, it sets into a hard mass with a good strength.
(3) Plaster of Paris : (CaSO4.1/2H2O) or (2CaSO4.H2O)
Preparation : Plaster of paris as mentioned above is prepared by the partial dehydration of gypsum at 390 K
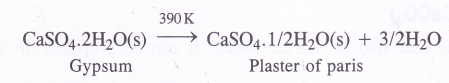
Temperature should not be allowed to rise beyond 390 K in order to check the formation of completely anhydrous CaSO4.
Properties :
- It is a white amorphous powder.
- When mixed with three times its weight of water, it forms a plastic mass which sets into a hard mass within ten to fifteen minutes. This is known as setting of plaster of paris. The setting is most probably because of rehydration back to gypsum.
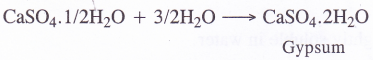
During setting, the mass undergoes a slight expansion (about 1 %) in volume. This helps plaster of paris to take the shape of any mould in which it is put.
Uses :
- Plaster of paris is commonly used in making moulds for pottery, ceramics etc.
- It is also used in surgical bandages for setting broken bones of the body.
- It is used for making statues, models, decorative materials and black board chalks.
Question 22.
Why are lithium salts commonly hydrated while those of other alkali metal ions are usually anhydrous ?
Answer:
In the lithium salts, the lithium ion (Li+) due to very small size gets readily hydrated on coming in contact with moisture (or water). Therefore, lithium salts are commonly hydrated. But the other alkali metal ions are comparatively big in size. They have therefore, lesser tendency to get hydrated. These salts are usually anhydrous.
Question 23.
Why is LiF almost insoluble in water while LiCl is soluble not only in water but also in acetone ?
Answer:
The low solubility of LiF in water is due to its very high lattice enthalpy (F~ ion is very small in size). On the other hand, in lithium chloride (LiCl), the lattice enthalpy is comparatively very small due to comparatively large size of CL ion. This means that the magnitude of hydration enthalpy is quite large. Therefore, lithium chloride dissolves in water and also in acetone due to dipolar attraction. (Acetone is polar in nature
Question 24.
Explain significance of sodium, potassium, magnesium and calcium as biological fluids.
Answer:
- Sodium and Potassium
Living beings need nearly 27 elements out of which about 15 are metals. Alkali metals (Na, K) and alkaline earth metals (Ca, Mg) are needed in major quantities. Minor quantities of other metals like Fe, Co, Cu, Zn, Mo, Ni, Al etc. are also needed in different organisms.
Both sodium and potassium in the form of ions are common and essential constituents of biological fluids. In a person weighing 70 kg, sodium and potassium are present to the extent of 90 g and 170 g respectively along with 5 g of iron and 0-06 g of copper.
Although both Na+ and K+ are chemically similar, they have different biological functions to perform. The Na+ ions are present in blood plasma and in the interstitial fluid which surrounds the cells. These ions take part in the transmission of nerve signals, regulate the flow of water across cell membranes and help in the transport of different sugars and amino acids into cells. The K+ ions present in abundance in the cell fluids, activate a variety of enzymes and also promote oxidation of glucose into ATP which is a source of energy. Both sodium and potassium are also responsible for the transmission of nerve signals.
The Na+ and K+ ions differ in concentration on the opposite sides of the cell membranes. For example, in blood plasma, the concentrations of Na+ and K+ ions are 143 milli mol/L and 5 milli mole/L respectively. However, within the red blood cells, the respective concentrations of these ions are 10 milli mol/L and 105 milli mol/L. This ratio is known as concentration gradient or ionic gradient. In order to maintain the concentration gradient in the cells, work has to be done. For this, a sodium potassium pump operates across the cell membranes and consumes nearly one third of the ATP used by a resting animal.
- Magnesium and Calcium
We have discussed earlier the biological importance of sodium and potassium ions. The ions of magnesium and calcium are also found in large proportions in biological fluids and they perform a variety of biological functions. An adult body contains about 25 g of magnesium and 200 g of calcium as compared to 5 g of iron and 0 06 g of copper.
Mg2+ ions are the constituent of chlorophyll, the green colouring pigment in plants. It absorbs photons from light and carries photosynthesis in plants. Ca2+ ions are the major constituent of our bones in the form as apatite Ca3(PO4)2. The deficiency of these ions occurs particularly in old age. Similarly, Ca2+ ions are a constituent of the enamel of our teeth as fluorapatite [3Ca3(P04)2 CaF2] They also play an important role in muscle contraction. These functions are related to the energy storage in biological systems in the form of pyrophosphate linkage. Both Mg2+ and Ca2+ act as catalysts in the formation of these linkages. The release of energy due to hydrolysis of pyrophosphate is controlled by Ca2+ ions. Calcium occurs mainly in milk. Vitamin D helps in the deposition of calcium in bones.
Question 25.
What happens when
- Sodium metal is dropped in water
- Sodium metal is heated in free supply of air
- Sodium peroxide dissolves in water ?
Answer:
(1) Sodium hydroxide is forced. The metal catches fire. Actually, the hydrogen evolved is highly combustible and it catches fire
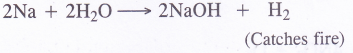
(2) Sodium peroxide is formed
2Na + O2 → Na2O2
(3) Oxygen gas is evolved
2Na2O2 + 2H2O —> 4NaOH + O2
Question 26.
(a) Why is LiF least soluble in water among the fluorides of alkali metals ?
(b) Justify the given order of mobilities of the alkali metal cations in aqueous solution :
Li+ < Na+ < K+ < Rb+ < Cs+
(c) Lithium is the only alkali metal which forms a nitride directly. Explain.
(d) E° for M2+(aq) —> M(s) (where M = Ca, Sr or Ba) is nearly constant. Discuss.
Answer:
(a) Lithium fluoride (LiF) is of covalent nature because of the high polarising power of Li+ ion due to its very small size and high effective nuclear charge. It distorts the electron cloud of the F– ion to the maximum as compared to the cations of other alkali metals. It is therefore, least soluble in water. On the other hand, the fluorides of other alkali metals are generally ionic and are water soluble.
(b) This is attributed to the hydration of the cation in water. As a result, size of the cation increases and its mobility decreases. Due to the smallest size, Li+ ion is hydrated to the maximum and exists as Li+ (aq) and has least mobility. Cs+ion due to least hydration exists as Cs+ (aq) has maximum mobility.
(c) Lithium is a very strong reducing agent. As a result, it directly exists as Cs+ (aq) combines with nitrogen to form its nitride (Li3N)
![]()
(d) The overall magnitude of reduction potential (E°) depends upon three factors. These are
- sublimation enthalpy
- ionisation enthalpy and
- hydration enthalpy.
In case of the metals listed, the overall magnitude of E° values remain almost the same. Therefore, these metals have almost same reducing strength
Question 27.
State as to why :
(a)A solution of Na2CO3 is alkaline.
(b)Alkali metals are prepared by the eletrolysis of their fused chlorides.
(c)Sodium is found to be more useful than potassium.
Answer:
(a)Sodium carbonate being a salt of strong base (NaOH) and weak acid (H2CO3), forms an alkaline solution upon hydrolysis
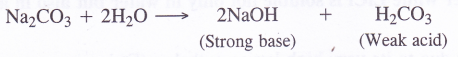
(b)Since alkali metals are highly reactive chemically, they react with water to evolve hydrogen gas. These are therefore, prepared by the electrolysis of their fused chlorides.
(c) Sodium is relatively more abundant than potassium. At the same time, it is also less reactive and its reactions with other substances can be better controlled.
Question 28.
Write the balanced equations for the reactions between :
(1) Na2O2 and water
(2) KO2 and water
(3) Na2O and CO2
aNS:
- 2Na2O2 + 2H2O → 4NaOH + O2
- 2KO2 + 2H2O → 2KOH + H2O2 + O2
- Na2O + CO2 → Na2CO3.
Question 29.
How would you explain the following :
- BeO is insoluble in water while BeSO4 is soluble.
- Ba(OH)2 is soluble in water while BaSO4 is almost insoluble.
- Lil is more soluble than KI in ethanol.
- NaHCO3 is known in the solid state but Ca(HCO3)2 is not isolated in the solid state.
Answer:
- The lattice enthalpy of BeO is higher as compared to BeSO4 because the size of O2- ion is very small while SO42- ion has bigger size. Since high lattice enthalpy opposes the solubility of a substance in water therefore, BeO is almost insoluble while BeSO4 is soluble in water.
- The size of the SO42- ion in BaSO4 is quite big as compared to that of OH– ion in Ba(OH)2. The SO42- ion has masked the Ba2+ ion in BaSO4 to a large extent with the result that the cation has a very little tendency to get hydrated. On the other hand, the OH– ion due to smaller size masks the
- Ba2+ ions to lesser extent which means that hydration energy released when Ba(OH)2 dissolves in water is quite large. Therefore, Ba(OH)2 readily dissolves in water while BaSO4 is almost insoluble.
- Lil is mainly covalent while KI has ionic nature. In fact, the size of Li+ ion is smaller than that of K+ ion and it polarises the electron cloud of I– ion to a greater extent. With the result, LiI dissolves in ethanol or ethyl alcohol (organic solvent) whereas KI is almost insoluble.
- NaHCO3 is less soluble in water than Ca(HCO3)2. Therefore, it can be precipitated from the solution while it is difficult to precipitate Ca(HCO3)2. Actually, Ca2+ ion has a greater tendency to get
hydrated than Na+ ion due to its small size and more magnitude of positive charge. Therefore Ca(HCO3)2 is more soluble in water than NaHCO3.
Question 30.
Which of the following alkali metals is having the least melting point ?
(a) Na (b) K (c) Rb (d)
Answer:
(d) Cesium (Cs) has the least m.p. due to its maximum size the least lattice enthalpy.
Question 31.
Which of the following alkali metals gives hydrated salts ?
(a) Li (b) Na (c) K (d)
Answer:
(a) The salts of lithium are generally hydrated because the size of the Li+ ion is very small. It has maximum hydration enthalpy.
Question 32.
Thermally the most stable alkaline earth metal carbonate is :
(a) MgCO3 (b) CaCO3 (c) SrCO3 (d) BaCO3.
Answer:
(d) Barium carbonate (BaCO3) is thermally the most stable. For more details, consult section 10.14.
We hope the NCERT Solutions for Class 11 Chemistry Chapter 10 The s-Block Elements (Alkali and Alkaline Earth Metals), help you. If you have any query regarding NCERT Solutions for Class 11 Chemistry Chapter 10 The s-Block Elements (Alkali and Alkaline Earth Metals), drop a comment below and we will get back to you at the earliest.