Atoms Class 12 MCQs Questions with Answers
Atoms Class 12 MCQ Chapter 12 Physics Question 1.
O2 molecule consists of two oxygen atoms. In the molecule, nuclear force between the nuclei of the two atoms
(A) is not important because nuclear forces are short-ranged.
(B) is as important as electrostatic force for binding the two atoms.
(C) cancels the repulsive electrostatic force between the nuclei.
(D) is not important because oxygen nucleus have equal number of neutrons and protons.
Answer:
(A) is not important because nuclear forces are short-ranged.
Explanation:
As we know that the nuclear forces is too much stronger. Only attractive force as compared to electrostatic repulsive force and nuclear force decreases to zero on increasing distance. So in case of oxygen molecule, the distance between atoms of oxygen is larger as compared to the distances between nucleons in a nucleus. So that, the force between the nuclei of two oxygen atoms is not important as nuclear forces are short-ranged forces.
Atoms MCQ Questions Class 12 Chapter 12 Question 2.
A set of atoms in an excited state decays.
(A) in general, to any of the states with lower energy.
(B) into a lower state only when excited by an external electric field.
(C) all together simultaneously into a lower state.
(D) to emit photons only when they collide.
Answer:
(A) in general, to any of the states with lower energy.
Explanation:
A set of atoms in an excited state decays in general to any of the states with lower energy.
![]()
Physics 12th MCQ Questions Chapter 12 Question 3.
Two H atoms in the ground state collide inelastically. The maximum amount by which their combined kinetic energy is reduced, is
(A) 10.20 eV
(B) 20.40 eV
(C) 13.6 eV
(D) 27.2 eV.
Answer:
(A) 10.20 eV
Explanation:
Total energy of two H-atom in ground state = 2(-13.6) = -27.2 eV The maximum amount by which their combined kinetic energy is reduced when any one H-atom goes into first excited state after the inelastic collision, that is, the total energy of two H-atom after inelastic collision:
E = \(\frac{13.6}{n^{2}}\) M + 13.6
= \(\frac{13.6}{n^{2}}\)+ 13.61 [For excited state (n = 2)]
= 3.4 4- 13.6 = 17.0 eV
So that the loss in kinetic energy due to inelastic collision will be,
= 27.2 -17.0 = 10.2 eV
Physics Class 12 MCQ Questions Chapter 12 Question 4.
Taking the Bohr radius as a0 = 53 pm, the radius of Li++ ion in its ground state, on the basis of Bohr’s model, will be about
(A) 53 pm.
(B) 27 pm.
(C) 18 pm.
(D) 13 pm.
Answer:
(C) 18 pm.
Explanation:
According to Bohr’s model of an atom, radius of an atom in its ground state is
r = \(\frac{r_{0}}{2}\)
where, r0 is Bohr’s radius and Z is atomic number.
As given that,
r0 = 53 pm and atomic number of Lithium atom is 3
so, r = \(\frac{53}{3}\) = 17.67 pm = 18 pm
![]()
Question 5.
The binding energy of a H-atom, considering an electron moving around a fixed nuclei (proton), is B = – \(\frac{m e^{4}}{8 n^{2} \varepsilon_{0}^{2} h^{2}}\) (m = electron mass). If one decides to work in a frame of reference where the electron is at rest, the proton would be moving around it. By similar arguments, the binding energy would be Me4
B = – \(\frac{m e^{4}}{8 n^{2} \varepsilon_{0}^{2} h^{2}}\) (M = proton mass) This last expression is not correct because
(A) n would not be integral.
(B) Bohr-quantisation applies only to electron
(C) the frame in which the electron is at rest is not inertial.
(D) the motion of the proton would not be in circular orbits, even approximately.
Answer:
(C) the frame in which the electron is at rest is not inertial.
Explanation:
In a hydrogen atom, electrons revolving around a fixed proton nucleus have some centripetal acceleration. So that, its frame of reference is non-inertial. In the frame of reference, where the electron is at rest, the given expression is not true as it forms the non- inertial frame of reference. As the mass of an electron is negligible as compared to proton, so the centripetal force cannot provide the electrostatic force, So the given expression is not true, as it forms non-inertial frame of reference due to me me< < < mP or centripetal force on Fe< < < FP.
Question 6.
The simple Bohr model cannot be directly applied to calculate the energy levels of an atom with many electrons. This is because
(A) of the electrons not being subject to a central force.
(B) of the electrons colliding with each other.
(C) of screening effects.
(D) the force between the nucleus and an electron will no longer be given by Coulomb’s law.
Answer:
(A) of the electrons not being subject to a central force.
Explanation:
The simple Bohr model cannot be directly applied to calculate the energy levels of an atom with many electrons because when we derive the formula for radius/energy levels, etc., we make the assumption that centripetal force is provided only by electrostatic force of attraction by the nucleus.
So that, this will only work for single electron atoms. In multi-electron atoms, there will also be repulsion due to other electrons. The simple Bohr model cannot be directly applied to calculate the energy levels of an atom with many electrons.
![]()
Question 7.
For the ground state, the electron in the H-atom has an angular momentum = h, according to the simple Bohr model. Angular momentum is a vector and hence there will be infinitely many orbits with the vector pointing in all possible directions. In actuality, this is not true,
(A) because Bohr model gives incorrect values of angular momentum.
(B) because only one of these would have a minimum energy.
(C) angular momentum must be in the direction of spin of electron.
(D) because electrons go around only in horizontal orbits.
Answer:
(A) because Bohr model gives incorrect values of angular momentum.
Explanation:
According to; Bohr’s second postulate of atomic model, angular momentum of revolving electron must be some integral multiple of \(\frac{h}{2π}\) So the Bohr’s model of atom does not give correct value of angular momentum.
Question 8.
Choose the correct alternative from the clues given at the end of each statement:
(A) The size of the atom in Thomson’s model is ………….. the atomic size in Rutherford’s model. (much greater than/no different from/much less than)
(B) In the ground state of ………….. electrons are in stable equilibrium, while in electrons always experience a net force. (Thomson’s model/Rutherford’s model)
(C) A classical atom based on ………….. is doomed to collapse. (Thomson’s model/Rutherford’s model)
(D) An atom has a nearly continuous mass distribution in ………….. but has a highly non-uniform mass distribution in (Thomson’s model/Rutherford’s model)
(E) The positively charged part of the atom possesses most of the mass in ………….. (Rutherford’s model/both the models)
Answer:
(D) An atom has a nearly continuous mass distribution in ………….. but has a highly non-uniform mass distribution in (Thomson’s model/Rutherford’s model)
Explanation:
(A) The sizes of the atoms taken in Thomson’s model is not different from the atomic size in Rutherford’s model.
(B) In the ground state of Thomson’s model, the electrons are in stable equilibrium, while in Rutherford’s model, electrons always experience a net force.
(C) A classical atom based on Rutherford’s model is doomed to collapse.
(D) An atom has a nearly continuous mass distribution in Thomson’s model, but has a highly non-uniform mass distribution in Rutherford’s model.
(E) The positively charged part of the atom possesses most of the mass in both the models.
![]()
Assertion And Reason Based MCQs (1 Mark each)
Directions: In the following questions, A statement of Assertion (A) is followed by a statement of Reason (R). Mark the correct choice as.
(A) Both A and R are true and R is the correct explanation of A
(B) Both A and R are true but R is NOT the correct explanation of A
(Q A is true but R is false
(D) A is false and R is true
Question 1.
Assertion (A): Bohr postulated that the electrons in stationary orbits around the nucleus do not radiate.
Reason (R): According to classical Physics, all moving electrons radiate.
Answer:
(B) Both A and R are true but R is NOT the correct explanation of A
Explanation:
Bohr postulated that electrons in stationary orbits around the nucleus do not radiate. This is true. According to classical Physics, the moving electrons radiate only when they jump from a higher energy orbit to the lower energy orbit. So, the reason is false.
Question 2.
Assertion (A): According to Rutherford, atomic model, the path of electron is parabolic.
Reason (R): Rutherford could not explain the stability of atom.
Answer:
(D) A is false and R is true
Explanation:
According to Rutherford, “the entire positive charge and most of the mass of the atom is concentrated in a small volume called the nucleus, with electrons revolving around the nucleus just as planets revolve around the Sun.” So the assertion is false.
The electron orbiting around the nucleus radiate energy. As a result, the radius of the orbit continuously decreases and the electron fall Into the nucleus. So, stability of atom is not explained. Hence the reason is true.
![]()
Question 3.
Assertion (A): In the a-particle scattering experiment, most of the a-particles pass undeviated.
Reason (R): Most of the space in the atom is empty.
Answer:
(A) Both A and R are true and R is the correct explanation of A
Explanation:
Most of the a-particles pass roughly in a straight line (within 1°) without deviation. This shows that no force is acting on them. So assertion is true. Most of the space in the atom is empty. Only 0.14% of a-particles are scattered more than 1°.
Question 4.
Assertion (A): Bohr model is not applicable for multi-electron model.
Reason (R): Bohr model cannot account for sublevel (s, p, d,f) orbitals and electron spin.
Answer:
(A) Both A and R are true and R is the correct explanation of A
Explanation:
Bohr model works well for H and He+ having one electron only. But it does not work for multi-electron atoms, since it cannot account for sublevel (s, p, d, f) orbitals and electron spin. So, assertion and reason both are true and reason explains the assertion.
![]()
Case-Based MCQs
Attempt any 4 sub-parts out of 5. Each sub-part carries 1 mark.
I. Read the following text and answer the following questions on the basis of the same:
Atomic Absorption Spectrometer:
The atomic absorption (AA) spectrometer is used to analyze metals at very low concentrations, typically in the parts per million (ppm) or parts per billion (ppb) ranges. A liquid sample containing dissolved material whose concentration is to be measured is aspirated into a thin, wide AA flame, or is introduced into a small carbon furnace which is heated to a high temperature. Basic Principle of AAS is the measurement of absorption of radiation by free atoms.
The total amount of absorption depends on the number of free atoms present and the degree to which the free atoms absorb the radiation. At the high temperature of the AA flame, the sample is broken down into atoms using an atomizer and it is the concentration of these atoms that is measured. Sample in the form of solution is used. It is broken up into a fine mist with the help of an atomizer. When the mist reaches the flame, the intense heat breaks up the sample into its individual atoms.
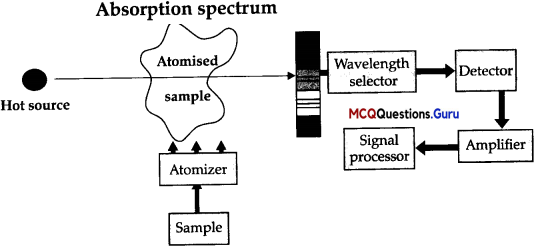
When a photon coming out from the hot source hits an atom and the energy of the photon is equal to the gap between two electron energy levels of the atom, then the electron in the lower energy level absorb the photon and jumps up to the higher energy level. If the photon energy does not correspond to the difference between two energy levels, then the photon will not be absorbed (it may be scattered away).
Hence in the spectrum, the wavelength corresponding to the absorbed photons is observed as black lines as shown in the following spectrum of Hydrogen. The dark lines correspond to the frequencies of light those have been absorbed by the sample element. Using this process, a source of photons (generally a white light) of various energies is used to obtain the absorption spectra of different materials and to identify them.
Question 1.
What is the basic principle of Atomic Absorption Spectrophotometer?
(A) Emission of photons when excited electron of an atom comes back to lower energy level.
(B) Absorption of photons when electrons at lower energy level jumps to a higher energy level.
(C) Emission of electrons from an atom at a very high temperature.
(D) Emission of electron when energetic photons bombard an atom.
Answer:
(B) Absorption of photons when electrons at lower energy level jumps to a higher energy level.
Explanation:
Basic principle of AAS is the 1 measurement of absorption of radiation by free atoms. When a photon hits an atom and the
energy of the photon is equal to the gap between two electron energy levels of the atom, the electron in the lower energy level absorb the photon and jumps up to the higher energy level.
![]()
Question 2.
What happens when a photon hits an atom and the energy of the photon is not equal to the gap between two electron energy levels of the atom?
(A) The photon is absorbed and the electron moves to an intermediate energy level.
(B) The photon is absorbed and the electron gets scattered
(C) The photon is not absorbed. It gets scattered.
(D) None of the above
Answer:
(C) The photon is not absorbed. It gets scattered.
Explanation:
When a photon hits an atom and the energy of the photon is equal to the gap between two electron energy levels of the atom, the electron in the lower energy level absorb the photon and jumps up to the higher energy level. If the photon energy does not correspond to the difference between two energy levels, then the photon will not be absorbed (it may be scattered away).
Question 3.
How the corresponding wavelength of the absorbed photon is represented in the absorption spectrum?
(A) By a black line
(B) By a white line
(C) By a black line in the lower wavelength range and by a white line in the higher wavelength range
(D) By a white line in the lower wavelength range and by a black line in the higher wavelength range
Answer:
(A) By a black line
Explanation:
In the spectrum, the wavelength corresponding to the absorbed photons is observed as black lines.
![]()
Question 4.
What should be the concentration of metal for analysis using Atomic Absorption Spectrometer?
(A) Very High concentration
(B) Very Low concentration
(C) Medium concentration
(D) Any concentration
Answer:
(B) Very Low concentration
Explanation:
The atomic absorption (AA) spectrometer is used to analyze metals at very low concentrations, typically in the parts per million (ppm) or parts per billion (ppb) ranges.
Question 5.
How the sample for analysis is driven to atomic state in AAS?
(A) At a very high temperature, the sample is driven to its gaseous state
(B) Using an atomizer and then intense heating.
(C) By rotating the solution of the sample at a very high speed.
(D) None of the above
Answer:
(B) Using an atomizer and then intense heating.
Explanation:
Sample in the form of solution is used. It is broken up into a fine mist with the help of an atomizer. When the mist reaches the flame, the intense heat breaks up the sample into its individual atoms.
II. Read the following text and answer the following questions on the basis of the same:
Spectrum Analysis and Astronomy Each element in the periodic table can appear in gaseous form and produce its own spectrum unique to that element. Hydrogen will not look like Helium, which will not look like carbon which will not look like iron… and soon. Astrophysists can identify what kinds of materials are present in stars from the analysis of star’s spectra. This type of study is called astronomical spectroscopy. The science of spectroscopy is quite sophisticated.
![]()
From spectrum lines analysis astrophysists can determine not only the element, but the temperature and density of that element in the star. The spectral line also can tell us about any magnetic field of the star. The width of the line can tell us how fast the material is moving. We can learn about winds in stars from this.
| Temperature (Kelvin) | Predominant radiation | Astronomical Examples |
| 600 K | Infrared | Planets, warm dust |
| 6,000 K | Optical | The photosphere of Sun and other stars |
| 60,000 K | UV | The photosphere of very hot stars |
| 600,000 K | Soft X-rays | The corona of the Sun |
| 6,000,000 K | X-rays | The coronae of active stars |
The shifting of spectral lines shift back and forth indicates that the star may be orbiting another star. The following table shows a rough guide for the relationship between the temperature of a star and the electromagnetic spectrum. sight. Edwin Hubble observed that more distant galaxies tended to have more red shifted spectra. This establishes the theory of expansion of the universe.
Question 1.
What is astronomical spectroscopy?
(A) Study of spectrum of star light and to identify its distance from Earth.
(B) Study spectrum of star light and to identify what kinds of elements are present in stars.
(C) Both (A) and (B)
(D) None of the above
Answer:
(B) Study spectrum of star light and to identify what kinds of elements are present in stars.
Explanation:
Astrophysists can identify what kinds of materials are present in stars from the analysis of star’s spectra. This type of study is called astronomical spectroscopy.
Question 2.
From the spectrum analysis the following information of a star can be obtained.
(A) Elements present, temperature
(B) magnetic field, density, mass
(C) distance of the star
(D) Both (A) and (B)
Answer:
(D) Both (A) and (B)
Explanation:
From spectrum lines analysis, astrophysists can determine not only the element, but the temperature and density of that element in the star. The spectral line also can tell us about any magnetic field of the star.
![]()
Question 3.
The lines in a star’s spectrum is found to shift back and forth. What conclusion may be drawn from this observation?
(A) The star may be orbiting another star
(B) There may be a storm in the star
(C) The star may be rotating at a very high speed
(D) None of the above
Answer:
(A) The star may be orbiting another star
Explanation:
The shifting of spectral lines shift I back and forth indicates that the star may be orbiting another star.
Question 4.
What may be the approximate temperature if soft X-rays are found predominantly in the spectrum?
(A) 60000 C
(B) 600000 C
(C) 60000 K
(D) 600000 K
Answer:
(D) 600000 K
Explanation:
From the table, we find that predominant presence of soft X-rays in the spectrum indicated that the temperature is 600000 K.
Question 5.
Which nature of spectrum establishes the theory of the expanding universe?
(A) Red-shift of spectrum
(B) Blue-shift of spectrum
(C) Back and forth movement of spectral lines
(D) None of the above
Answer:
(A) Red-shift of spectrum
Explanation:
If the spectrum of a star is red or blue shifted, then it can be used to infer its velocity along the line of sight. Edwin Hubble observed that more distant galaxies tended to have more red-shifted spectra. This establishes the theory of expansion of the universe.