Dual Nature of Radiation and Matter Class 12 MCQs Questions with Answers
Dual Nature Of Radiation And Matter MCQ Class 12 Question 1.
Consider a beam of electrons (each electron with energy E0) incident on a metal surface kept in an evacuated chamber. Then,
(A) no electrons will be emitted as only photons can emit electrons.
(B) electrons can be emitted but all with an energy, E0.
(C) electrons can be emitted with any energy, with a maximum of E0 – ø (ø is the work function).
(D) electron can be omitted with energy, with a maximum of E0.
Answer:
(D) electron can be omitted with energy, with a maximum of E0.
Eiplanation:
If a beam of electrons of having energy E0 is incident on a metal surface kept in an evacuated chamber.
The electrons can be emitted with maximum energy E0 (due to elastic collision) and with any energy less than E0, when part of incident energy of electron is used in liberating the electrons from the surface of metal. –
MCQ On Dual Nature Of Matter And Radiation Class 12 Question 2.
The wavelength of a photon needed to remove a proton from a nucleus which is bound to the nucleus with 1 MeV energy is nearly
(A) 1.2 nm
(B) 1.2 x 10-3nm
(C) 1.2 x 10-6 nm
(D) 1.2 x 10 nm
Answer:
(B) 1.2 x 10-3nm
Explanation:
Energy of the photon must be equal to the binding energy of proton So, energy of photon = 1 MeV
= 106 x 1.6 x 10-19
λ = \(\frac{h c}{E}=\frac{6.63 \times 10^{-34} \times 3 \times 10^{8}}{1.6 \times 10^{-13}}\) = \(\frac{6.63 \times 3}{1.60}\) x 10-26 + 13
= \(\frac{19.89}{1.60}\) x 10-13 = 12.4 x 10-13 = 1.24 x 10-1 x 10-13
= 1.24 x 10-9 x 10-3 =1.24 x 10-3 nm
![]()
Dual Nature Of Matter And Radiation Class 12 MCQ Chapter 11 Question 3.
The phenomenon which shows quantum nature of electromagnetic radiation is:
(A) photoelectric effect.
(B) Tyndall effect.
(C) interference.
(D) reflection and refraction.
Answer:
(A) photoelectric effect.
Dual Nature Of Radiation And Matter Chapter 11 Class 12 MCQ Question 4.
Kinetic energy of electrons emitted in photoelectric effect is
(A) directly proportional to the intensity of incident light.
(B) inversely proportional to the intensity of incident line.
(C) independent of the intensity of incident light.
(D) independent of the frequency of light.
Answer:
(C) independent of the intensity of incident light.
Explanation:
KE = hv – ø
So, KE is independent of intensity of incident light.
MCQ On Dual Nature Of Matter And Radiation Class 12 Question 5.
Threshold wavelength of a photoelectric emission from a material is 600 nm. Which of the following illuminating source will emit photoelectrons?
(A) 400 W, infrared lamp
(B) 10 W, ultraviolet lamp
(C) 100 W, ultraviolet lamp
(D) Both (B) & (C)
Answer:
(D) Both (B) & (C)
Explanation:
The incident wavelength should be less than threshold wavelength for photoelectric emission. IR has wavelength more than 600 nm. UV has wavelength less than 600 nm. So, photoelectrons emitted when illuminated by UV lamp either 100 W or 10 W
![]()
MCQ Of Dual Nature Of Radiation And Matter Class 12 Question 6.
Photoelectrons emitted from a metal have
(A) different speeds starting from 0 to certain maximum.
(B) same kinetic energy.
(C) same frequency.
(D) Both (B) & (C)
Answer:
(A) different speeds starting from 0 to certain maximum.
Explanation:
When a photon strikes a metal surface, the surface electrons come out with maximum speed and maximum kinetic energy. But if the electron emission takes place from inner side of metal, then some energy of the electron is lost due to collision with other electrons and so their speed becomes less. So, ultimately the electrons come out with different speeds.
Dual Nature Of Matter And Radiation MCQ Class 12 Chapter 11 Question 7.
At stopping potential, the kinetic energy of emitted photoelectron is
(A) minimum
(B) maximum.
(C) zero
(D) cannot de predicted
Answer:
(C) zero
MCQs On Dual Nature Of Radiation And Matter Class 12 Question 8.
Photons are
(A) electrically neutral and not deflected by electric or magnetic field.
(B) electrically neutral and deflected by magnetic field.
(C) electrically charged and not deflected by electric or magnetic field.
(D) electrically charged and not deflected by electric field.
Answer:
Option (A) is correct
![]()
Class 12 Physics Chapter 11 MCQ Questions Question 9.
A particle is dropped from a height H. The de-Broglie wavelength of the particle as a function of height is proportional to
(A) H
(B) H1/2
(C) H0
(D) H-1/2
Answer:
(D) H-1/2
Explanation:
Velocity v, of freely falling body after falling from a height H, will be :
H = υ = \(\sqrt{2gh}\)
We know that de-Broglie wavelength, λ = \(\frac {h}{p}\) ?
λ = \(\frac {h}{mυ}\) = \(\frac{h}{m \sqrt{2 g H}}\)
h, m, and g are constant
∴ \(\frac{h}{m \sqrt{2 g}}\) is constant ⇒λ ∝
Question 10.
A proton, a neutron, an electron and an a-particle have same energy. Then, their de-Broglie wavelengths compare as
(A) λp = λn > λe > λ∝
(B) λ∝ < λp = λn> λe
(C) λe < λp = λn > λ∝
(D) λe = λp = λn = λ∝
Answer:
(B) λ∝ < λp = λn> λe
Explanation:
Matter waves (de-Broglie waves) According to de-Broglie, a moving material particle sometimes acts as a wave and sometimes as a particle.
De- Broglie wavelength:
λd = \(\frac {h}{p}\)
Ed = En = Ee = Ex
K.E. = K = \(\frac {1}{2}\)mυ2
2K = mυ2
2Km = m2υ2
2mK = p2
\(\sqrt{2 m \mathrm{~K}}\) = p
∴ λd = \(\frac {h}{p}\)
λd = \(\frac{h}{\sqrt{2 m \mathrm{~K}}}\)
or λd = \(\frac{h}{\sqrt{2 m \mathrm{~K}}}\)[as h and E(K.E) is const.]
∴ λ ∝ \(\frac{1}{\sqrt{m}}\)
ma >mp = mn >me
∴ λa <λp = λn < λe
![]()
Question 11.
An electron is moving with an initial velocity υ = υ0 \(\hat{\mathbf{i}}\) and is in a magnetic field B = B0\(\hat{\mathbf{j}}\). Then, its de-Broglie wavelength
(A) remains constant.
(B) increases with time.
(C) decreases with time.
(D) increases and decreases periodicalLy.
Answer:
(A) remains constant.
Explanation:
Given, υ = υ0 and B = B0\(\hat{\mathbf{j}}\) Magnetic force on moving electron
= -e[υ0i x B0j] = – eυ0B0 k
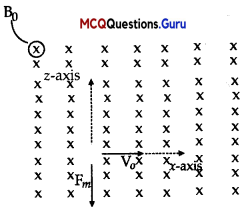
So, the force is perpendicular to y and B both as the force is perpendicular to the velocity. So, the magnitude will not change V or mv (p = mv momentum) so, the de-Broglie wavelength remains same.
Question 12.
An electron (mass m) with an initial velocity υ υ j (υ0 > 0) is in an electric field E = – E0\(\hat{\mathbf{i}}\) (E0 = constant > 0). Its de-Broglie wavelength at time t is given by
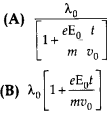
(C) decreases with time.
(D) increases and decreases periodicalLy.
Answer:
Option (A) is correct.
Explanation:
The wave associated with moving particle is called matter wave or de-Broglie wave and it propagates in the form of wave packets with group velocity.. According to de-Broglie theory the wavelength of de-Broglie wave is given by
λ = \(\frac{h}{p}=\frac{h}{m v}=\frac{h}{\sqrt{2 m E}}\)
As initial velocity of the electron is υ0\(\hat{\mathbf{i}}\), the initial de-Broglie wavelength of electron,
λ = \(\frac{h}{m v_{0}}\) …………(1)
Electrostatic force on electron in electric field is,
\(\vec{F}\)e = -e\(\vec{E}\) = -e[-E0\(\hat{\mathbf{i}}\)] = eEz0\(\hat{\mathbf{i}}\)
Acceleration of electron, \(\vec{a}\) = \(\frac{\vec{F}}{m}\) = \(\frac{e E_{0} \hat{j}}{m}\)
Velocity of the electron after time t,
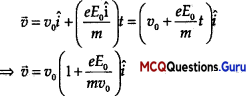
de-Broglie wavelength associated with electron at time t is λ = \(\frac{h}{m v}\)
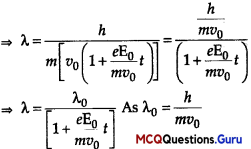
![]()
Question 13.
An electron (mass m) with an initial velocity υ = υ0\(\hat{\mathbf{i}}\) is in an electric field E = E0 \(\hat{\mathbf{j}}\) if λ0 = \(\frac{h}{m v_{0}}\) its de-Broglie wavelength at time t is given by

Answer:
Option (C) is correct.
Explanation:
According to the problem de-Broglie wavelength of electron at time
t = 0 is λ0 = \(\frac{h}{m v_{0}}\)
Electrostatic force on electron in electric field is
\(\vec{F}\)e = -e\(\vec{E}\) = -eE0\(\hat{\mathbf{j}}\)
The acceleration of electron, \(\vec{a}\) = \(\frac{\overrightarrow{\mathrm{F}}}{m}\) = \(\frac{e \mathrm{E}_{0}}{m} \hat{j}\)
it is acting along negative y-axis.
The initial velocity of electron along x-axis,
\(v_{x_{0}}\) = v0\(\hat{\mathbf{i}}\)
This component of velocity will remain constant as there is no force on electron in this direction. Now considering y-direction, initia! velodty of electron along y-axis, \(v_{y_{0}}\) = 0 Velocity of electron after time t along y-axis
υy = 0 + \(\left(-\frac{e \mathrm{E}_{0}}{m} \mathrm{~J}\right)\)t = \(-\frac{e \mathrm{E}_{0}}{m} \hat{t} \hat{j}\)
Magnitude of velocity of electron after time t is

de-Broglie wavelength, λ0 = \(\frac{h}{m v_{0}}\)
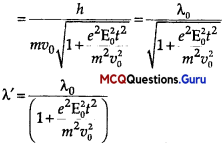
Question 14.
The ratio of dc-Brogue wavelength associated with two electrons accelerated through 25 V and 36 V is
(A) 25/36
(B) W25
(C) 5/6
(D) 6/5
Answer:
(D) 6/5
Explanation:
λ ∝ 1/ √V
∴λ 1/λ2 = √(V2 /V1) = 6/5
Question 15.
Which of the following graphs shows the variation of de-Broglie wavelength with potential through which a particle of charge q and mass m is accelerated?
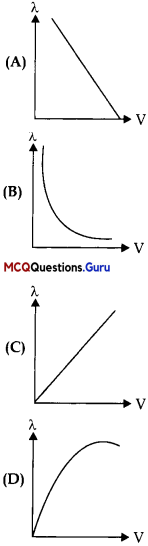
Answer:
Option (B) is correct.
Assertion And Reason Based MCQs (1 Mark each)
Directions: In the following questions, A statement of Assertion (A) is followed by a statement of Reason (R). Mark the correct choice as.
(A) Both A and R are true and R is the correct explanation of A
(B) Both A and R are true but R is NOT the correct explanation of A
(C) A is true but R is false
(D) A is false and R is true
![]()
Question 1.
Assertion (A): The energy (E) and momentum (p) of a photon are related as p = E/c
Reason (R): The photon behaves like a particle.
Answer:
(A) Both A and R are true and R is the correct explanation of A
Explanation:
Energy= E = hc/λ Momentum = P = h/λ. So, P = E/c
This is true only when photon has a particle nature.
So, assertion and reason both are true and reason properly explains the assertion.
Question 2.
Assertion (A): Photoelectric effect demonstrates the particle nature of light.
Reason (R): The number of photoelectrons is proportional to the frequency of light.
Answer:
(C) A is true but R is false
Explanation:
Photoelectric effect demonstrates the particle nature of light. So assertion is true. Number of emitted photoelectrons depends upon the intensity of light. So reason is false.
Question 3.
Assertion (A): Kinetic energy of photoelectrons emitted by a photosensitive surface depends upon the frequency of incident photon.
Reason (R): The ejection of electrons from metallic surface is possible with frequency of incident photon below the threshold frequency.
Answer:
(C) A is true but R is false
Explanation:
K.E. depends upon the frequency. So assertion is true. Photoelectron emitted when frequency of incident radiation is more than the threshold frequency. So reason is also false.
![]()
Question 4.
Assertion (A): Photosensitivity of a material is high if its work function is low.
Reason (R): Work function = hf0, where /0 is threshold frequency.
Answer:
(B) Both A and R are true but R is NOT the correct explanation of A
Question 5.
Assertion (A): de-Broglie equation is significant for microscopic particles.
Reason (R): de-Broglie wavelength is inversely proportional to the mass of a particle when velocity is kept constant.
Answer:
(A) Both A and R are true and R is the correct explanation of A
Explanation:
de-Broglie wavelength, λ = h/mυ h and v remaining constant, λ ∝ 1/m So, as the mass of the particle becomes smaller and smaller the de-Broglie wavelength of the particle becomes more and more significant. Hence, assertion and reason both are true and reason explains the assertion properly.
Question 6.
Assertion (A): de-Broglie wavelength of a gas molecule is inversely proportional to the square root of temperature.
Reason (R): The root mean square velocity of gas molecules depends on temperature.
Answer:
(A) Both A and R are true and R is the correct explanation of A
Explanation:
υRMS = \(\sqrt{\frac{3 R T}{M}}\)
So, υRMS ∝ √T
Again de-Broglie wavelength, λ = h/mυ
So, λ ∝ 1/√T
Hence, assertion and reason both are true and reason explains the assertion
![]()
Question 7.
Assertion (A): Resolving power of electron microscope increases as electron is accelerated through higher voltage.
Reason (R): de-Broglie wavelength of electron decreases as accelerating voltage of electron increases.
Answer:
(A) Both A and R are true and R is the correct explanation of A
Explanation:
de-Broglie wavelength is given by
λ = \(\frac{h}{\sqrt{2 m e V}}\)
So, as accelerating potential (V) increases, the wavelength decreases.
Resolving power of microscope = \(\frac{2 \mu \sin \beta}{1.22 \lambda}\)
So, as wavelength (X) decreases, resolving power increases. So, assertion and reason both are true and reason explains the assertion.
Case-Based MCQs
Attempt any 4 sub-parts out of 5. Each sub-part carries 1 mark.
I. Read the following text and answer the following questions on the basis of the same:
Photocell:
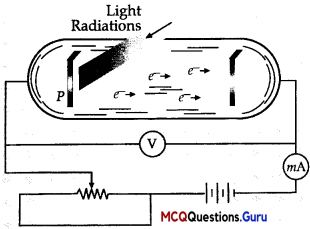
A photocell is a technological application of the photoelectric effect. It is a device whose electrical properties are affected by light. It is also sometimes called an electric eye. A photocell consists of a semi-cylindrical photo-sensitive metal plate C (emitter) and a wire loop A (collector) supported in an evacuated glass or quartz bulb. It is connected to the external circuit having a high-tension battery B and micro ammeter (pA) as shown in the Figure.
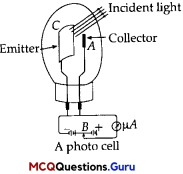
Sometimes, instead of the plate C, a thin layer of photosensitive material is pasted on the inside of the bulb. A part of the bulb is left clean for the light to enter it. When light of suitable wavelength falls on the emitter C, photoelectrons are emitted. These photoelectrons are drawn to the collector A. Photocurrent of the order of a few microampere can be normally obtained from a photo cell. A photocell converts a change in intensity of illumination into a change in photocurrent. This current can be used to operate control systems and in light measuring devices.
Question 1.
Photocell is an application of
(A) thermoelectric effect.
(B) photoelectric effect.
(C) photoresistive effect.
(D) None of the above
Answer:
(B) photoelectric effect.
Explanation:
Photocell is a technological application of the photoelectric effect
![]()
Question 2.
Photosensitive material should be connected to
(A) -ve terminal of the battery.
(B) +ve terminal of the battery.
(C) any one of (A) or (B).
(D) connected to ground.
Answer:
(A) -ve terminal of the battery.
Explanation:
Photosensitive material used as emitter should be connected to -ve terminal of the battery so that the emitted electrons are repelled by emitter and collected by collector.
Question 3.
Which of the following statement is true?
(A) The photocell is totally painted black.
(B) A part of the photocell is left clean.
(C) The photocell is completely transparent.
(D) A part of the photocell is made black.
Answer:
(B) A part of the photocell is left clean.
Explanation:
A part of the bulb is left clean for the light to enter in it.
Question 4.
The photocurrent generated is in the order of
(A) ampere
(B) milliampere
(C) microampere
(D) None of the above
Answer:
(C) microampere
Explanation:
Photocurrent of the order of a few microampere can be normally obtained from a photocell.
Question 5.
A photocell converts a change in of incident light into a change in
(A) intensity, photovoltage
(B) wavelength, photovoltage
(C) frequency, photocurrent
(D) intensity, photocurrent
Answer:
(D) intensity, photocurrent
Explanation:
A photocell converts a change in intensity of illumination into a change in photocurrent.
![]()
II. Read the following text and answer the following questions on the basis of the same:
Electron Microscope
Electron microscopes use electrons to illuminate a sample. In Transmission Electron Microscopy (TEM), electrons pass through the sample and illuminate film or a digital camera. Resolution in microscopy is limited to about half of the wavelength of the illumination source used to image the sample. Using visible light the best resolution that can be achieved by microscopes is about —200 nm. Louis de Broglie showed that every particle or matter propagates like a wave. The wavelength of propagating electrons at a given accelerating voltage can be determined by
l = \(\frac{h}{\sqrt{2 m_{e} v}}\)
Thus, the wavelength of electrons is calculated to be 3.88 pm when the microscope is operated at 100 keV, 2. 74 pm at 200 keV and 2.24 pm at 300 keV. However, because the velocities of electrons in an electron microscope reach about 70% the speed of light with an accelerating voltage of 200 keV, there are relativistic effects on these electrons. Due to this effect, the wavelength at 100 keV, 200 keV and 300 keV in electron microscopes is 3.70 pm, 2.51 pm and 1.96 pm, respectively.
Anyhow, the wavelength of electrons is much smaller than that of photons (2.5 pm at 200 keV). Thus if electron wave is used to illuminate the sample, the resolution of an electron microscope theoretically becomes unlimited. Practically, the resolution is limited to -0.1 nm due to the objective lens system in electron microscopes. Thus, electron microscopy can resolve subcellular structures that could not be visualized using standard fluorescences microscopy.
Question 1.
In electron microscope, electron is used:
(A) to charge the sample.
(B) to. clean the sample.
(C) to illuminate the sample.
(D) All of the above
Answer:
(C) to illuminate the sample.
Explanation:
Electrons as wave is used in electron microscopes to illuminate a sample since it enhances the resolving power.
![]()
Question 2.
Who showed that electron also propagates like a wave?
(A) Louis de Broglie
(B) Albert Einstein
(C) Philipp Lenard
(D) Wilhelm Ludwig Franz Hallwachs
Answer:
(A) Louis de Broglie
Explanation:
Louis de Broglie showed that every particle or matter propagates like a wave.
Question 3.
Why electron as wave is used in electron microscope to illuminate the sample?
(A) The wavelength of electrons as wave is much larger than that of photons, hence resolution is much better.
(B) The wavelength of electrons as wave is much smaller than that of photons, hence resolution is much better.
(C) Electron as wave wave is much brighter than normal light and hence resolution is much better.
(D) Speed of electron as wave wave is greater than the speed of light and hence offers better resolution.
Answer:
(B) The wavelength of electrons as wave is much smaller than that of photons, hence resolution is much better.
Explanation:
Using visible light, the best resolution that can be achieved by microscopes is about – 200 nm. The wavelength of electrons as wave is much smaller than that of photons as wave (2.5 pm at 200 keV). Thus if electron as wave is used to illuminate the sample, the resolution of an electron microscope theoretically becomes unlimited. Practically, the resolution is limited to – 0.1 nm,
Question 4.
As the accelerating voltage increases, the wavelength of electron as wave
(A) decreases.
(B) increases.
(C) remains same.
(D) upto 100 keV increases and then decreases.
Answer:
(A) decreases.
Explanation:
λ = \(\frac{h}{\sqrt{2 m e V}}\)
So, as V increases, λ decreases.
![]()
Question 5.
Wavelength of electron as wave at accelerating voltage 200 kev is
(A) 2.5 nm
(B) 2.5 mm
(C) 2.5 pm
(D) 2.5 pm
Answer:
(C) 2.5 pm