The p-Block Elements Class 12 MCQs Questions with Answers
P Block Elements Class 12 MCQ Chapter 7 Question 1.
Which of the following statements is wrong?
(A) Single N-N bond is stronger than the single P-P bond.
(B) PH3 can act as a ligand in the formation of coor-dination compound with transition elements.
(C) NO2 is paramagnetic in nature.
(D) Covalency of nitrogen in N2O5 is four.
Answer:
(A) Single N-N bond is stronger than the single P-P bond.
Explanation:
N-N single bond is weaker than P-P bond due to smaller size of N as compared to P. Smaller size of N leads to smaller N-N bond length. Because of larger size of P atom, P-P bond length is more and lone pair-lone pair repulsion between P atoms is less which makes the P-P bond stronger than N-N bond.
P Block MCQ Class 12 Chapter 7 Question 2.
Which of the following elements can be involved in pπ-dπ bonding?
(A) Carbon
(B) Nitrogen
(C) Phosphorus
(D) Boron
Answer:
(C) Phosphorus
Explanation:
pπ-dπ bonding is present in phosphorus due to the presence of vacant d-orbitals and in carbon (C), nitrogen (N) and boron (B) do not have d orbitals.
![]()
P Block MCQ Class 12 Chapter 7 Question 3.
Bond dissociation enthalpy of E-H (E = element) bond is given below. Which of the compounds will act as strongest reducing agent?

(A) NH3
(B) PH3
(C) AsH2
(D) SbH3
Answer:
(D) SbH3
Explanation:
The strongest reducing agent is SbH3 due to the presence of minimum bond enthalpy.
MCQ On P Block Elements Class 12 Chapter 7 Question 4.
On heating with concentrated NaOH solution in an inert atmosphere of CO2 white phosphorus gives a gas. Which of the following statement is incorrect about the gas?
(A) It is highly poisonous and has smell like rotten fish.
(B) It’s solution in water decomposes in the pres¬ence of light.
(C) It is more basic than NH3.
(D) It is less basic than NH3.
Answer:
(C) It is more basic than NH3.
Explanation:
PH3 is less basic than NH3
P4+ 3NaOH + 3H20 → PH3 + 3NaH2PO2(Phosphine)
MCQ Of P Block Elements Class 12 Chapter 7 Question 5.
A brown ring is formed in the ring test for NO3 ion. It is due to the formation of:
(A) [Fe(H2O)5 (NO)]2+
(B) FeSO4 NO2.
(C) [Fe(H2O)4(NO)2]2+
(D) FeSO4.HNO3.
Answer:
(A) [Fe(H2O)5 (NO)]2+
Explanation:
When freshly prepared solution of ferrous sulphate (FeSO4) is added in a solution containing NO3– ion, formation of a brown- coloured complex will take place. This is called as brown ring test of nitrate. Hence, two moles of ammonia will produce two moles of NO.
NO3– + 3Fe2+ + 4H+ → NO + 3Fe3+ + 2H2O
[Fe(H2O)6]2+ + NO → [Fe(H2O)5(NO)]2+(Brown ring) + H2O
![]()
P Block Elements MCQ Class 12 Chapter 7 Question 6.
Hot cone. H2SO4 acts as moderately strong oxidising agent. It oxidises both metals and non-metals. Which of the following element is oxidised by cone. H2S04 into two gaseous products?
(A) Cu
(B) S
(C) C
(D) Zn
Answer:
(C) C
Explanation:
C + 2H2SO4 → CO2+ 2SO2 + 2H2O Hot concentrated sulphuric acid should be used to oxidise carbon to carbon dioxide.
P Block Class 12 MCQ Chapter 7 Question 7.
Which of the following are peroxoacids of sulphur?
(A) H2SO5 and H2S2O8
(B) H2SO5 and H2S2O7
(C) H2S2O7 and H2S2O8
(D) H2S2O6 and H2S2O7
Answer:
(A) H2SO5 and H2S2O8
Explanation:
H2SO5 and H2S2O5 Peroxymonosulphuric acid and Peroxydis sulphuric acid are peroxoacids of sulphur.
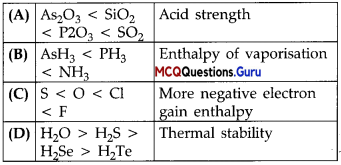
Class 12 P Block MCQ Chapter 7 Question 8.
Which of the following statements are correct for SO2 gas?
(A) It acts as bleaching agent in moist conditions.
(B) Its molecule has linear geometry.
(C) It can be prepared by the reaction of dilute H2SO4 with metal sulphide.
(D) All of the above
Answer:
(A) It acts as bleaching agent in moist conditions.
Explanation:
SO2 acts as a bleaching agent under moist conditions. SO2(g) + 2H2O → H2SO2 + 2[H] SO2 is oxidized to sulphuric acid and releases nascent hydrogen which bleaches the material. But this is a temporary as atmospheric oxygen reoxides the bleached matter after some time.
![]()
P Block Elements MCQ Class 12 Chapter 7 Question 9.
Which of the following orders are correct as per the properties mentioned against each?
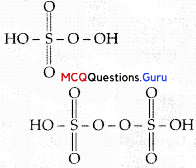
Answer:
Option (A, D) is correct.
Explanation:
Acidic strength of oxides in group: Decreases down the group and increases along a period from left to right. Thermal stability of hydrides of group 16 decreases down the group.
Class 12 Chemistry P Block MCQ Chapter 7 Question 10.
Which, of the following statements are correct?
(A) S – S bond is present in H2S2O8.
(B) In peroxosulphuric acid (H2SO5) sulphur is in +6 oxidation state.
(C) Iron powder along with Al2O3 and K2O is used as a catalyst in the preparation of NH3 by Haber’s process.
(D) Change in enthalpy is positive for the prepara-tion of SO3 by catalytic oxidation of S02.
Answer:
(B) In peroxosulphuric acid (H2SO5) sulphur is in +6 oxidation state.
Explanation:
In H2SO5, there is a peroxo-linkage:
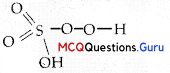
(O in peroxide linkage has oxidation state -1)
P Block Elements Class 12 MCQs Chapter 7 Question 11.
In which of the following reactions cone. H2SO4 is used as an oxidising reagent?
(A) CaF2 + H2SO4 → CaSO4 + 2HF
(B) 2HI + H2SO4 → I2 +SO2 + 2H2O
(C) Cu + 2H2SO4 → CuSO4 + SO2+ 2H2O
(D) NaCl + H2SO4 → NaHSO4 + HCl
Answer:
(B) 2HI + H2SO4 → I2 +SO2 + 2H2O
(C) Cu + 2H2SO4 → CuSO4 + SO2+ 2H2O
Explanation:
In given four reactions, option (B) and (C) represent oxidising behaviour of H2SO4 that oxidising agent reduces itself as oxidation state of central atom decreases. The reaction is given below:
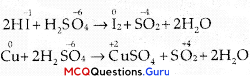
![]()
Class 12 Chemistry Chapter 7 MCQ Question 12.
Which of the following is not tetrahedral in shape?
(A) NH4+
(B) SiCl4
(C) SF4
(D) SO42-
Answer:
(C) SF4
Explanation:
SF4 has trigonalbi-pyramidal structure.
MCQs On P Block Elements Class 12 Chapter 7 Question 13.
Which of the following does not react with oxygen directly?
(A) Zn
(B) Ti
(C) Pt
(D) Fe
Answer:
(C) Pt
Explanation:
Platinum (Pt) is an inert metal and does not react very easily. All other elements, Zn, Ti and Fe, are quite reactive. Flence, Pt does not react with oxygen directly.
P Block Elements MCQs Class 12 Chapter 7 Question 14.
Affinity for hydrogen decreases in the group from fluorine to iodine. Which of the halogen acids should have highest bond dissociation enthalpy?
(A) H-F
(B) HC1
(C) HBr
(D) HI
Answer:
(A) H-F
Explanation:
F being smallest has the shortest H – F bond and therefore HF has the highest bond dissociation energy.
The P Block Elements Class 12 MCQ Chapter 7 Question 15.
Reduction potentials of some ions are given below. Arrange them in decreasing order of oxidising power.
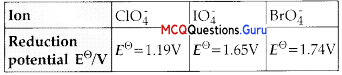
(A)ClO–4>IO–4> BrO–4
(B) IO–4>BrO–4>CIO–4
(C) BrO–4> IO–4> CIO–4
(D) BrO–4> CIO–4> IO–4
Answer:
(C) BrO–4> IO–4> CIO–4
Explanation:
The higher the reduction potential, the higher is its tendency to get reduced. Hence, the order of their oxidising power is:
BrO4> IO4> CIO4
![]()
Class 12 P Block Elements MCQ Chapter 7 Question 16.
Which of the following is iso-electronic pair?
(A) ICl2,CIO2
(B) BrO–4, BrF+2
(C) CIO2, BrF
(D) CN–, O3
Answer:
(B) BrO–4, BrF+2
Explanation:
(A) ICl2 = 53 + 2 x 17 = 87
ClO2 = 17 + 16 = 33
(B) BrO2 = 35+ 2×8 + 1= 52
BrF+2 = 35 +9 x 2 – 1 = 52
(C) ClO = 17 + 16 = 33
BrF = 35 + 9 = 44
(D) CN– = 6 + 7 + 1 = 14
O3 = 8 x 3 = 24
Class 12 Chemistry Chapter P Block MCQ Question 17.
A black compound of manganese reacts with a halogen acid to give greenish yellow gas. When excess of this gas reacts with NH3 an unstable trihalide is formed. In this process the oxidation state of nitro-gen changes from:
(A) -3 to +3.
(B) -3 to 0
(C) – 3 to +5.
(D) 0 to – 3.
Answer:
(A) -3 to +3.
Explanation:
MnO3 + 4HCl + MnCl2 + 2H2O + Cl2 (Greenish yellow gas)
NH3 + 3Cl2 → NCl3 + 3HCl
When excess of chlorine reacts with ammonia then NCl2 and HCl will form. In this reaction on left-hand side chlorine has (-3) oxidation state and on the right-hand chlorine has (+3) oxidation state.
![]()
P-Block Elements Class 12 MCQ Chapter 7 Question 18.
Which of the following statements are true?
(A) Only types of interactions between particles of noble gases are due to weak dispersion forces.
(B) Ionisation enthalpy of molecular oxygen is very close to that of xenon.
(C) Hydrolysis of XeF6 is a redox reaction.
(D) Xenon fluorides are not reactive.
Answer:
(A) Only types of interactions between particles of noble gases are due to weak dispersion forces.
Explanation:
Weak dispersion forces are present between particles of noble gases. Ionisation enthalpy of molecular oxygen is very close to that of xenon.
P Block Elements Class 12 MCQ Questions Question 19.
Which of the following statements are correct?
(A) Among halogens, radius ratio between iodine and fluorine is maximum.
(B) Leaving F – F bond, all halogens have weaker X – X bond than X – X’ bond in inter-halogens.
(C) Among inter-halogen compounds maximum number of atoms ate present in iodine fluoride.
(D) Inter-halogen compounds are more reactive than halogen compounds.
Answer:
(B) Leaving F – F bond, all halogens have weaker X – X bond than X – X’ bond in inter-halogens.
Explanation:
In case of halogens radius ratio between iodine and fluorine is maximum radius because iodine has maximum radius while fluorine has minimum radius. Also, due to highest ratio maximum numbers oi atom”, are present in iodine fluoride. Inter-halogen compounds are more reactive than halogen compounds because A-B bond of dissimilar halogen is weaker than A-A or B-B bond of halogens.
Chemistry Class 12 P Block Elements MCQ Question 20.
Which one of the following does not exist?
(A) XeOF4
(B) NeF2
(C) XeF2
(D) XeF6
Answer:
(B) NeF2
Explanation:
Xe has least ionisation energy among the noble gases and hence it forms chemical compounds with oxygen and fluorine, however, Ne cannot form compounds with oxygen and fluorine so NeF2 does not exist
![]()
Question 21.
In the preparation of compounds of Xe, Bartlett had taken O2 + PtF+6 as a base compound. This is because
(A) both O2 and Xe have same size.
(B) both O2 and Xe have same electron gain enthalpy.
(C) both O2 and Xe have same ionisation enthalpy.
(D) both Xe and O2 are gases.
Answer:
(C) both O2 and Xe have same ionisation enthalpy.
Explanation:
In the preparation of compounds of Xe, Bartlett had taken O2 + PlF6 as a base compound. This is because both O2 and Xe have almost same ionisation enthalpy.
Question 22.
Which of the following statements are true?
(A) Only types of interactions between particles of noble gases are due to weak dispersion forces.
(B) Hydrolysis of XeF6 is a redox reaction.
(C) Xenon fluorides are not reactive.
(D) None of the above.
Answer:
(B) Hydrolysis of XeF6 is a redox reaction.
Explanation:
Only types of interactions between particles of noble gases are due to weak dispersion forces.
Question 23.
When XeF4 is partially hydrolysed, it yields
(A) XeSO3
(B) XeOF2
(C) XeOF4
(D) XeF2
Answer:
(B) XeOF2
Explanation:
Partial hydrolysis of XeF4gives oxyfluorides, XeOF4 and XeO2F2.
XeF6 + H2O → XeOF4 + 2HF (Xenon oxytotrafluoride)
XeF6+ 2H2O → Xe02F2 + 4HF ? (Xenon dioxvdiflnoride)
![]()
Question 24.
Which of the following reactions is an example of redox reaction?
(A) XeF4 + O2F2 → XeF6 + O2
(B) XeF2 + PF5 → [XeF] + [PF6]–
(C) XeF6 + H2O → XeOF4 + 2HF
(D) XeF6 + 2H2O → XeO2F2 + 2HF
Answer:
(A) XeF4 + O2F2 → XeF6 + O2
Explanation:
XeF4 + O2F2 → XeF6 + O2
Question 25.
Complete the following reaction: Xe + PtF6 →
(A) Xe + PtF6 → XeF4 + PtF2
(B) Xe + PtF6 → XeF6 + Pt
(C) Xe + PtF6 → Xe+ + [PtF6]–
(D) Xe + PtF6 → Xe02F4 + Pt
Answer:
(C) Xe + PtF6 → Xe+ + [PtF6]–
Explanation:
Xe + PtF6 → Xe+ + [PtF6]–
Question 26.
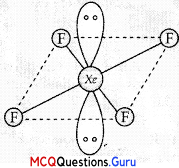
The shape of XeF4 is
(A) tetr&hedral
(B) square planar
(C) pyramidal
(D) linear
Answer:
(B) square planar
Explanation:
XeF4 is square planar in structure.
Question 27.
Main source of helium is
(A) Air
(B) Radium
(C) Monazite
(D) Water
Answer:
(C) Monazite
Explanation:
Monazite is the main source of Helium
![]()
Assertion And Reason Based MCQs
Directions:
In the following questions, A statement of Assertion (A) is followed by a statement of Reason (R). Mark the correct choice as.
(A) Both A and R are true and R is the correct explanation of A
(B) Both A and R are true but R is NOT the correct explanation of A
(C) A is true but R is false
(D) A is false and R is True
Question 1.
Assertion (A): N2 is less reactive than P4.
Reason (R): Nitrogen has more electron gain en¬thalpy than phosphorus.
Answer:
(C) A is true but R is false
Explanation:
Due to high bond dissociation energy of triple bond between the two N atoms, nitrogen (N) is less reactive than P4 and its electron gain enthalpy is less than phosphorus.
Question 2.
Assertion (A): HNO3 makes iron passive.
Reason (R): HNO3 forms a protective layer of ferric nitrate on the surface of iron.
Answer:
(C) A is true but R is false
Explanation:
HNO3 makes iron passive and its passivity is attained by formation of a thin film of oxide on iron.
Question 3.
Assertion (A): Bismuth forms only one well char-acterised compound in +5 oxidation state.
Reason (R): Elements of group-15 form compounds in +5 oxidation state.
Answer:
(B) Both A and R are true but R is NOT the correct explanation of A
Explanation:
Elements of group-15 form; compounds in +5 oxidation stale. Bismuth forms f: only one well characterised compound in +5| oxidation state which is BiF3. Due to inert pair effect bismuth exhibit +3 oxidation state and only forms trihalides. But due to small size and high electronegativity of fluorine, Bismuth forms BiF5
![]()
Question 4.
Assertion (A): NaCl reacts with concentrated H2SO4 to give colourless fumes with pungent smell. But on adding MnO2 the fumes become greenish yellow.
Reason (R): MnO2 oxidises HCl to chlorine gas which is greenish yellow.
Answer:
(A) Both A and R are true and R is the correct explanation of A
Explanation:
Colourless fumes of hydrochloric acid (HCl) become greenish yellow because MnO2 oxidises HCl to chlorine gas.
Question 5.
Assertion (A): Both rhombic and monoclinic sulphur exist as Sg but oxygen exists as O2.
Reason (R): Oxygen forms pπ-pπ multiple bond due to small size and small bond length but pπ-pπ bonding is not possible in sulphur.
Answer:
Option (A) is correct.
Explanation:
Sulphur (S) exists as S8 but oxygen forms pπ-pπ multiple bonds which is not present in S.
Question 6.
Assertion (A): NaCl reacts with concentrated H2SO4 to give colourless fumes with pungent smell. But on adding MnOz the fumes become greenish yellow.
Reason (R): MnO2 oxidises HCl to chlorine gas which is greenish yellow
Answer:
(A) Both A and R are true and R is the correct explanation of A
Explanation:
Colourless fumes of hydrochloric acid(HCl) because greenish yellow because MnO2 oxidises HCl to chlorine gas.
![]()
Question 7.
Assertion (A): SF6 cannot be hydrolysed but SF4 can be.
Reason (R): Six atoms in SF6 prevent the attack of H2O on sulphur atom of SF6.
Answer:
(A) Both A and R are true and R is the correct explanation of A
Explanation:
SF6 is sterically protected due to presence of six atoms around S atom which prevents the attack of H2O on SF4 can be
Question 8.
Assertion (A): H2O a liquid and H2S a gas.
Reason (R): Water molecules are held by H-bonds while in H2S molecules no such interactions are present between molecules.
Answer:
(C) A is true but R is false
Explanation:
Due to small size and high electronegativilv of oxygen, water is highly associated with inlertnolecular hydrogen bonding but molecules of H2S are held together by van der Waal’s Ibices of attraction. Hence, H2O is a liquid and GS gas.
Question 9.
Assertion (A): HI cannot be prepared by the reaction of KI with concentrated H2SO4.
Reason (R): HI has lowest H – X bond strength among halogen acids.
Answer:
(B) Both A and R are true but R is NOT the correct explanation of A
Explanation:
Both statements are correct but are independent of each other. HI cannot be prepared by the reaction of KI with ? concentrated H2SO4 as it results in the formation – of HI which further oxidizes to I2 as H2SO4 is a strong oxidizing agent.
![]()
Question 10.
Assertion (A): F2 is a strong oxidizing agent.
Reason (R): Electron gain enthalpy of fluorine is less negative.
Answer:
(B) Both A and R are true but R is NOT the correct explanation of A
Explanation:
Fluorine is the best oxidising agent, because it has more reduction potential (more ability to lose the electrons) which is attributed to its high electro negativity.
Question 11.
Assertion (A): F2 has lower bond dissociation energy than Cl2.
Reason (R): Flourine is more electronegative than chlorine.
Answer:
(D) A is false and R is True
Explanation:
F2 has higher bond dissociation enthalpy than Cl2.
Question 12.
Assertion (A): F2 has lower reactivity.
Reason (R): F – F bond has low Abond H°.
Answer:
(D) A is false and R is True
Explanation:
Fluorine is the maximum reactive because of low bond dissociation enthalpy.
Question 13.
Assertion(A): Group 18 gases exhibit very high ionisation enthalpy.
Reason (R): They have a stable electronic configuration.
Answer:
(A) Both A and R are true and R is the correct explanation of A
Explanation:
Group 18 gases exhibit very high; ionisation enthalpv because they have a stable; electronic configuration.
![]()
Question 14.
Assertion(A): The noble gases are inactive.
Reason(R): These gases have a closed shell structure.
Answer:
(A) Both A and R are true and R is the correct explanation of A
Explanation:
The noble gases are inactive as they have a closed shell structure.
Question 15.
Assertion(A): Helium diffuses through most commonly used laboratory materials.
Reason(R): This gas has a very low melting point.
Answer:
(C) A is true but R is false
Explanation:
Helium diffuses through most commonly used laboratory materials which is an unusual property of this gas.
Question 16.
Assertion (A): Helium used in diving apparatus.
Reason (R): Helium is very less soluble in blood.
Answer:
(A) Both A and R are true and R is the correct explanation of A
Explanation:
Helium used in diving apparatus 1 because of its low solubility in blood.
![]()
Case-Based MCQs
I. Read the passage given below and answer the following questions:
In spite of the predictions of stable noble gas compounds since at least 1902, unsuccessful attempts at their synthesis gave rise to the widely held opinion that noble gases are not only noble but also inert. It was not until 1962 that this dogma was shattered when Bartlett in Canada published the first stable noble gas compound Xe PtF6.
This discovery triggered a worldwide frenzy in this area, and within a short time span many new xenon, radon, and krypton compounds were prepared and characterized. The recent discoveries show the ability of xenon to act as a ligand . The discovery by Seppelt’s group that more than one xenon atom can attach itself to a metal center which in the case of gold leads to surprisingly stable Au- Xe bonds. The bonding in [AuXe4]2+ involves 4 Xe ligands attached by relatively strong bonds to a single Au(II) center in a square planar arrangement with a Xe-Au bond length of about 274 pm This discovery provides not only the first example of multiple xenon ligands but also represents the first strong metal – xenon bond.
Question 1.
In the complex ion [AuXe4]2+, Xe acts as:
(A) central atom
(B) ligand
(C) chelating agent
(D) electrophile
Answer:
(A) central atom
Question 2.
Hybridisation shown by Au in [AuXe4]2+ is:
(A) sp3
(B) sp3d
(C) sp3d2
(D) sp2
Answer:
(B) sp3d
Question 3.
Compounds of noble gases except are known.
(A) Krypton
(B) Radon
(C) Helium
(D) Xenon
Answer:
(C) Helium
![]()
Question 4.
Xe is a ligand
(A) ambidentate
(B) bidentate
(C) unidentate
(D) hexadentate
Answer:
(C) unidentate
II. Read the passage given below and answer the following questions:
In the last 10 years much has been learned about the molecular structure of elemental sulfur. It is now known that many different types of rings are sufficiently metastable to exist at room temperature for several days. It is known that at high temperature, the equilibrium composition allows for a variety of rings and chains to exist in comparable concentration, and it is known that at the boiling point and above, the vapour as well as the liquid contains small species with three, four, and five atoms.
The sulfur atom has the same number of valence electrons as oxygen. Thus, sulfur atoms S2 and S3 have physical and chemical properties analogous to those of oxygen and ozone. S2 has a ground state of 38 σ3S2 σ*3s2a3 pz2π3p x 2 = π3py2π*3px1 = π* 3py1. S3 , thiozone has a well known uv spectrum, and has a bent structure, analogous to its isovalent molecules O3, SO2, and S2O.
The chemistry of the two elements, sulphur and oxygen, differs because sulfur has a pronounced tendency for catenation. The most frequently quoted explanation is based on the electron structure of the atom. Sulfur has low-lying unoccupied 3d orbitals, and it is widely believed that the 4s and 3d orbitals of sulfur participate in bonding in a manner similar to the participation of 2s and 2p orbitals in carbon. In the following questions, a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices on the basis of the above passage.
(A) Assertion and reason both are correct statements and reason is correct explanation for assertion.
(B) Assertion and reason both are correct statements but reason is not correct explanation for assertion.
(C) Assertion is correct statement but reason is wrong statement.
(D) Assertion is wrong statement but reason is correct statement.
Question 1.
Assertion (A): Sulphur belongs to same group in the periodic table as oxygen.
Reason (R): S2 has properties analogous to O2.
Answer:
(B) Assertion and reason both are correct statements but reason is not correct explanation for assertion.
Question 2.
Assertion (A): Thiozone has bent structure like ozone.
Reason (R): Ozone has a lone pair which makes the molecule bent.
Answer:
(B) Assertion and reason both are correct statements but reason is not correct explanation for assertion.
![]()
Question 3.
Assertion (A): S2 is paramagnetic in nature
Reason (R): The electrons in π*3px and π*3py orbitals in S2 are unpaired.
Answer:
(A) Assertion and reason both are correct statements and reason is correct explanation for assertion.
Question 4.
Assertion (A): Sulphur has a greater tendency for catenation than oxygen.
Reason (R): 3d and 4s orbitals of Sulphur have same energy.
Answer:
(C) Assertion is correct statement but reason is wrong statement.
III. Read the given passage and answer the questions (i) to (iv) that follow:
The halogens have the smallest atomic radii in their respective periods. The atomic radius of fluorine is extremely small. All halogens exhibit -1 oxidation state. They are strong oxidising agents and have maximum negative electron gain enthalpy. Among halogens, fluorine shows anomalous behaviour in many properties. For example electronegativity and ionisation enthalpy are higher for fluorine than expected whereas bond dissociation enthalpy, m.p. and b.p. and electron gain enthalpy are quite lower than expected. Halogens react with hydrogen to give hydrogen halides (HX) and combine amongst themselves to form a number of compounds of the type XX’, XX’3, XX’5and XX’7 called inter halogens.
Question 1.
Why halogens have maximum negative electron gain enthalpy?
Answer:
Halogens have only seven electrons in their valence shell. So they require only one electron to attain a noble gas configuration. Hence they have maximum electron gain enthalpy.
Question 2.
Why fluorine shows anomalous behaviour as compared to other halogens?
Answer:
(i) It has smallest in size.
(ii) Very high electronegativity.
(iii) Absence of d-orbitals.
(iv) dissociation enthalpy in molecular form is least. (Any one)
![]()
Question 3.
Arrange the hydrogen halides (HF to HI) in the decreasing order of their reducing character.
Answer:
HI > HBr > HCl > HF
Question 4.
Why fluorine is a stronger oxidizing agent than chlorine?
Answer:
Because fluorine has greater E0 value (2.87V) than chlorine (1.36V).
Question 5.
What are the sizes of X and X’ in the interhalogen compounds?
Answer:
Size of X is greater than X’.