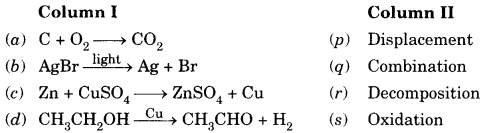Check the below NCERT MCQ Questions for Class 10 Science Chapter 2 Acids, Bases and Salts with Answers Pdf free download. MCQ Questions for Class 10 Science with Answers were prepared based on the latest exam pattern. We have Provided Acids, Bases and Salts Class 10 Science MCQs Questions with Answers to help students understand the concept very well. https://mcqquestions.guru/mcq-questions-for-class-10-science-chapter-2/
You can refer to NCERT Solutions for Class 10 Science Chapter 2 Acids, Bases and Salts to revise the concepts in the syllabus effectively and improve your chances of securing high marks in your board exams.
Acids, Bases and Salts Class 10 MCQs Questions with Answers
Acid Bases And Salts Class 10 MCQ Question 1.
What happens when a solution of an acid is mixed with a solution of a base in a test tube?
(i) The temperature of the solution increases
(ii) The temperature of the solution decreases
(iii) The temperature of the solution remains the same
(iv) Salt formation takes place
(a) (i) only
(b) (i) and (iii)
(c) (ii) and (iii)
(d) (i) and (iv)
Answer
Answer: (d) (i) and (iv)
Acids Bases And Salts Class 10 MCQ Question 2.
An aqueous solution turns red litmus solution blue. Excess addition of which of the following solution would reverse the change?
(a) Baking powder
(b) Lime
(c) Ammonium hydroxide solution
(d) Hydrochloric acid
Answer
Answer: (d) Hydrochloric acid
Class 10 Science Chapter 2 MCQ Question 3.
Two aqueous solutions P and Q have pH of 5 and 13 respectively. The correct inference is that:
(a) solution P is of HCl and Q is of NH4OH
(b) solution P is of CH3COOH and Q is of Ca(OH)2
(c) solution P is of HNO3 and Q is of NH4OH
(d) solution P is of CH3COOH and Q is of NaOH
Answer
Answer: (d) solution P is of CH3COOH and Q is of NaOH
Acid Base And Salts Class 10 MCQ Question 4.
The pH of a solution is 7. How can you increase its pH?
(a) By adding a small amount of acid
(b) By adding a small amount of base.
(c) By adding a small amount of salt.
(d) By passing carbon dioxide gas through it.
Answer
Answer: (b) By adding a small amount of base.
Class 10 Acids Bases And Salts MCQ Question 5.
Sodium carbonate is a basic salt because it is a salt of
(a) strong acid and strong base
(b) weak acid and weak base
(c) strong acid and weak base
(d) weak acid and strong base
Answer
Answer: (d) weak acid and strong base
MCQ Of Acid Base And Salts Class 10 Question 6.
Which gas is evolved when acids react with metals?
(a) O2
(b) CO2
(c) H2
(d) N2
Answer
Answer: (c) H2
Ch 2 Science Class 10 MCQ Question 7.
A sample of soil is mixed with water and allowed to settle. The clear supernatant solution turns the pH paper yellowish-orange. Which of the following would change the colour of this pH paper to greenish- blue?
(a) Lemon juice
(b) Vinegar
(c) Common salt
(d) An antacid
Answer
Answer: (d) An antacid
Class 10 Science Ch 2 MCQ Question 8.
Which of the following gives the correct increasing order of acidic strength?
(a) Water < Acetic acid < Hydrochloric acid
(b) Water < Hydrochloric acid < Acetic acid
(c) Acetic acid < Water < Hydrochloric acid
(d) Hydrochloric acid < Water < Acetic acid
Answer
Answer: (a) Water < Acetic acid < Hydrochloric acid
MCQ Questions For Class 10 Science Chemistry Chapter 2 Question 9.
If a few drops of a concentrated acid accidentally spills over the hand of a student, what should be done?
(a) Wash the hand with saline solution.
(b) Wash the hand immediately with plenty of water and apply a paste of sodium hydrogencarbonate.
(c) After washing with plenty of water and apply solution of sodium hydroxide on the hand.
(d) Neutralise the acid with a strong alkali.
Answer
Answer: (b) Wash the hand immediately with plenty of water and apply a paste of sodium hydrogencarbonate.
Acids Bases And Salts MCQ Question 10.
Farmers neutralise the effect of acidity of the soil by adding
(a) slaked lime
(b) gypsum
(c) caustic soda
(d) baking soda
Answer
Answer: (a) slaked lime
MCQ Questions For Class 10 Science Chapter 2 Question 11.
A teacher gave two test tubes to the students, one containing water and the other containing sodium hydroxide. She asked them to identify the test tube containing sodium hydroxide solution. Which one of the following can be used for the identification?
(a) Blue litmus
(b) Red litmus
(c) Sodium carbonate solution
(d) Dilute hydrochloric acid
Answer
Answer: (b) Red litmus
Chapter 2 Science Class 10 MCQ Question 12.
One of the constituents of baking powder is sodium hydrogencarbonate, the other constituent is
(a) hydrochloric acid
(b) tartaric acid
(c) acetic acid
(d) sulphuric acid
Answer
Answer: (b) tartaric acid
Class 10 Chemistry Chapter 2 MCQ Question 13.
Increase in the OH– ion concentration, leads to
(a) an increases in the pH of solution
(b) a decrease in the pH of the solution
(c) doesn’t alter the pH of the solution
(d) decreases the basic strength of the solution
Answer
Answer: (a) an increases in the pH of solution
Class 10 Acid Base And Salt MCQ Question 14.
Which of the following statements is correct about an aqueous solution of an acid and of a base?
(i) Higher the pH, stronger the acid
(ii) Higher the pH, weaker the acid
(iii) Lower the pH, stronger the base
(iv) Lower the pH, weaker the base
(a) (i) and (iii)
(b) (ii) and (iii)
(c) (i) and (iv)
(d) (ii) and (iv)
Answer
Answer: (iv) Lower the pH, weaker the base
MCQ On Acids Bases And Salts Class 10 Question 15.
A solution has turned the colour of red litmus paper to blue. The pH of the solution is approximately:
(a) 2
(b) 5
(c) 7
(d) 10
Answer
Answer: (d) 10
Class 10 Chapter 2 Science MCQ Question 16.
The pH of three solutions X, Y and Z is 6, 4 and 8 respectively. Which of the following is the correct order of acidic strength?
(a) X > Y > Z
(b) Z > Y > X
(c) Y > X > Z
(d) Z > X > Y
Answer
Answer: (c) Y > X > Z
Question 17.
Which one of the following can be used as an acid-base indicator by a visually impaired student?
(a) Litmus
(b) Turmeric
(c) Vanilla essence
(d) Petunia leaves
Answer
Answer: (c) Vanilla essence
Question 18.
What is gastric acid present in the stomach composed of?
(a) Hydrochloric acid
(b) Sulphuric acid
(c) Nitric acid
(d) Lactic acid
Answer
Answer: (a) Hydrochloric acid
Question 19.
Which of the following is acidic in nature?
(a) Lime juice
(b) Human blood
(c) Lime water
(d) Antacid
Answer
Answer: (a) Lime juice
Question 20.
When the solution of an acid is diluted, what will be the change in pH of the solution?
(a) pH of the solution remains the same
(b) pH of the solution will increase
(c) pH of the solution decreases
(d) pH of the solution climbs to 7
Answer
Answer: (a) pH of the solution remains the same
Question 21.
When the stopper of a bottle containing colourless liquid was removed, the bottle gave a smell like that of vinegar. The liquid in the bottle could be
(a) Hydrochloric acid solution
(b) Sodium hydroxide solution
(c) Acetic acid solution
(d) Saturated sodium bicarbonate solution
Answer
Answer: (a) Hydrochloric acid solution
Question 22.
Which of the following is not a mineral acid?
(a) Hydrochloric acid
(b) Citric acid
(c) Sulphuric acid
(d) Nitric acid
Answer
Answer: (b) Citric acid
Question 23.
Which among the following is not a base?
(a) NaOH
(b) KOH
(c) NH4OH
(d) C2H5OH
Answer
Answer: (d) C2H5OH
Question 24.
To protect tooth decay, one is advised to brush the teeth regulary. The ingredient of the paste which checks tooth decay is:
(a) acidic
(b) basic
(c) neutral
(d) corrosive
Answer
Answer: (b) basic
Question 25.
Match the chemical substances given in Column (A) with their appropriate application given in Column (B).
| Column I | Column II |
| (A) Bleaching powder | (i) Preparation of glass |
| (B) Baking soda | (ii) Production of H2 and Cl2 |
| (C)Washing soda | (iii) Decolourisation |
| (D) Sodium chloride | (iv) Antacid |
(a) A-(ii), B-(i), C-(iv), D-(iii)
(b) A-(iii), B-(ii), C-(iv), D-(i)
(c) A-(iii), B-(iv), C-(i), D-(ii)
(d) A-(ii), B-(iv), C-(i), D-(iii)
Answer
Answer: (c) A-(iii), B-(iv), C-(i), D-(ii)
Fill in the blanks
1. A solution turns red litmus blue, its pH is likely to be …………….
Answer
Answer: greater than 7
2. Plaster of Paris is made from …………….
Answer
Answer: gypsum
3. Phenolphthalein is ……………. in acidic solution.
Answer
Answer: colourless
4. An example of double salt is …………….
Answer
Answer: Mohr’s salt
5. Bleaching powder can be prepared by reacting …………….
Answer
Answer: slaked lime with chlorine
6. Hardness of water is removed by …………….
Answer
Answer: washing soda
7. ……………. is a deliquescent solid.
Answer
Answer: Caustic soda
8. The colour of methyl orange in basic medium is …………….
Answer
Answer: yellow
9. Boric acid is a ……………. acid.
Answer
Answer: monobasic
10. The process of formation of salt and water when an acid reacts with a base is called …………….
Answer
Answer: neutralisation
Match the following columns
1.
| Column I | Column II |
| (a) Sodium carbonate | (P) Fire-proofing material |
| (b) Plaster of Paris | (q) Use for faster cooking |
| (c) Bleaching powder | (r) Softening hard water |
| (d) Baking soda | (s) Textile industry |
Answer
Answer:
| Column I | Column II |
| (a) Sodium carbonate | (r) Softening hard water |
| (b) Plaster of Paris | (P) Fire-proofing material |
| (c) Bleaching powder | (s) Textile industry |
| (d) Baking soda | (q) Use for faster cooking |
2.
| Column I | Column II |
| (a) Baking soda | (p) NaCl |
| (b) Plaster of Paris | (q) NaHCO3 |
| (c) Washing soda | (r) CaSO4 . 1/2 H2O. |
| (d) Common salt | (s) Na2CO3 |
Answer
Answer:
| Column I | Column II |
| (a) Baking soda | (q) NaHCO3 |
| (b) Plaster of Paris | (r) CaSO4 . 1/2 H2O. |
| (c) Washing soda | (s) Na2CO3 |
| (d) Common salt | (p) NaCl |
3.
| Column I | Column II |
| (a) Mixed salts | (p) CaCO3 MgCO3 |
| (b) Double salts | (q) NaKCO3 |
| (c) Basic salts | (r) NaHSO4 |
| (d) Acidic salts | (s) CH3COONa |
Answer
Answer:
| Column I | Column II |
| (a) Mixed salts | (q) NaKCO3 |
| (b) Double salts | (p) CaCO3 MgCO3 |
| (c) Basic salts | (s) CH3COONa |
| (d) Acidic salts | (r) NaHSO4 |
Complete the crossword below.
Across
4. A synthetic indicator
6. Substance formed by hydration of plaster of Paris
7. A herbaceous plant with stinging hairs
8. Solution with pH less than 7
Down
1. Gas released by chemical reaction of zinc and sodium hydroxide
2. Reaction between acid and base
3. A substance that changes red litmus to blue
5. Medicine used during indigestion
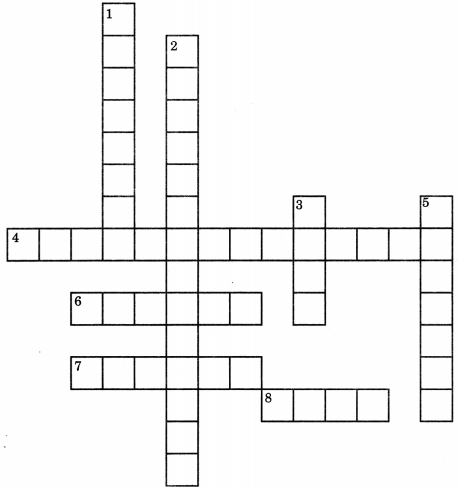
Answer
Answer:
Down:
1. Hydrogen
2. Neutralisation
3. Base
5. Antacid
Across:
4. Phenolphthalein
6. Gypsum
7. Nettle
8. Acid
We hope the given NCERT MCQ Questions for Class 10 Science Chapter 2 Acids, Bases and Salts with Answers Pdf free download will help you. If you have any queries regarding Acids, Bases and Salts CBSE Class 10 Science MCQs Multiple Choice Questions with Answers, drop a comment below and we will get back to you soon.