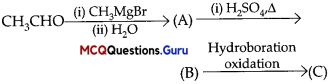
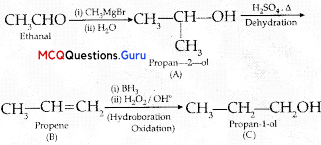
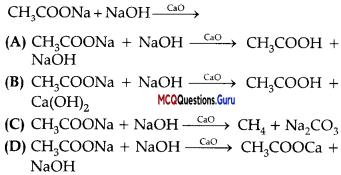
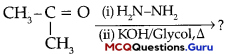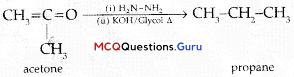Sexual Reproduction in Flowering Plants Class 12 MCQ Questions With Answers
Class 12 Biology Chapter 2 MCQ Question 1.
Among the terms listed below, those that of are not technically correct names for a floral whorl are :
(i) Androecium
(ii) Carpel
(iii) Corolla
(iv) Sepal
(A) (i) and (iv)
(B) (iii) and (iv)
(C) (ii) and (iv)
(D) (i) and (ii)
Answer:
(C) (ii) and (iv)
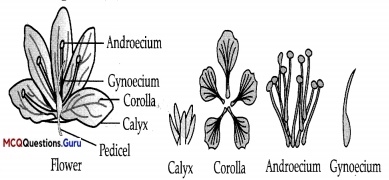
Explanation :
All the four whorls of the plant with their relative position in flower can be indicated through following diagram. Sepals collectively form a whorl, called as calyx while technically the carpel is known as gynoecium. The floral whorls formed by petals and stamens are called as corolla and androecium, respectively.

Sexual Reproduction In Flowering Plants Class 12 MCQ Question 2.
The embryo sac is related to ovule as is related to an anther.
(A) Stamen
(B) Filament
(C) Pollen grain
(D) Androecium
Answer:
(C) Pollen grain
Explanation :
The pollen grains represent the male gametophytes. As the anthers mature and dehydrate, the microspores dissociate from each other and develop into pollen grains. So, embryo sac is to ovule as pollen grain is to an ther
Chapter 2 Biology Class 12 MCQs Question 3.
In a typically complete, bisexual and hypogynous flower the arrangement of floral whorls on the thalamus from the outermost to the innermost is:
(A) Calyx, corolla, androecium and gynoecium
(B) Calyx, corolla, gynoecium and androecium
(C) Gynoedum, androecium, corolla and calyx
(D) Androecium, gynoecium, corolla and calyx
Answer:
(A) Calyx, corolla, androecium and gynoecium
Explanation :
In a typically complete, bisexual, and hypogynous flower the arrangement of floral whorls on the thalamus from the outer-most to the innermost is as follows :
(i) Calyx: It is the outermost whorl of sepals.
(ii) Corolla: It is a whorl of petals inside the calyx.
(iii) Androecium: It is a whorl of stamens inside the corolla.
(iv) Gynoecium: It is a whorl of pistils (in the center of the flower forming innermost whorls).
Chapter 2 Biology Class 12 MCQ Reproduction Question 4.
A dicotyledonous plant bears flowers but never produces fruits and seeds. The most probable cause for the above situation is:
(A) Plant is dioecious and bears only pistillate flowers
(B) Plant is dioecious and bears both pistillate and staminate flowers
(C) Plant is monoecious
(D) Plant is dioecious and bears only staminate flowers
Answer:
(D) Plant is dioecious and bears only staminate flowers
Explanation:
In dioecious plants, the unisexual male flower is staminate, that is, bearing stamens only,while the female is pistillate or bearing pistils only. For the production of fruits and seeds fertilization must take place, which is possible only in the presence of both male and female flowers. When the plant is dioecious, it will give rise to the following situations :
(i) If the plant is dioecious and bears only pistillate flowers, fertilization can take place with the help of pollinators.
(ii) If the plant is dioecious and bears only staminate flowers, fertilization cannot take place, because female gamete is non-motile which can’t reach the male gamete in order to fuse with it. When the plant is monoecious, that is, carrying both stamen and pistil together it may lead to self-fertilization and production of seed.
Chapter 2 Class 12 Biology MCQ Question 5.
The outermost and innermost wall layers of microsporangium in an anther are respectively:
(A) Endothecium and tapetum
(B) Epidermis and endodermis
(C) Epidermis and middle layer
(D) Epidermis and tapetum
Answer:
(D) Epidermis and tapetum
Explanation :
The outermost and innermost wall layers of microsporangium in an anther are respectively, epidermis and tapetum. A typical microsporangium is generally surrounded by four-wall layers, that is, the epidermis, (outermost protective layer), I endothecia, (middle fibrous layers) and the I tapetum (innermost nutritive layer).

Biology Chapter 2 Class 12 MCQs Question 6.
During microsporogenesis, meiosis occurs in:
(A) Endothecium
(B) Microspore mother cells
(C) Microspore tetrads
(D) Pollen grains.
Answer:
(B) Microspore mother cells
Explanation :
During microsporogenesis, meiosis occurs in microspore mother cells. As the anther develops, the microspore mother cells of the sporogenous tissue undergo meiotic divisions to form microspore tetrads. The micro-spore tetrad after dehydration is separated into pollen grains.
Biology Class 12 Chapter 2 MCQs Question 7.
From among the sets of terms given below, identify those that are associated with the gynoecium.
(A) Stigma, ovule, embryo sac, placenta
(B) Thalamus, pistil, style, ovule
(C) Ovule, ovary, embryo sac, tapetum
(D) Ovule, stamen, ovary, embryo sac
Answer:
(A) Stigma, ovule, embryo sac, placenta
Explanation :
Gynoecium indicates the female reproductive part of the flower which consists of pistil. Each pistil has three parts, that is, stigma,style, and ovary. Inside the ovarian cavity, the placenta is located. Arising from the placenta there are the megasporangia, commonly called ovules. The functional megaspore undergoing the meiotic division develops into the female gametophyte or embryo sac. Thalamus, tapetum and stamen are not a part of gynoecium. Thalamus is the part of flower which forms the base on which all the floral whorls rest up on. Tapetum is the innermost nutritive layer or microsporangium and stamens are male reproductive part (androecium) of plant.
Class 12 Bio Ch 2 MCQ Question 8.
From the statements given below choose the option that are true for a typical female gametophyte of a flowering plant:
(i) It is 8-nucleate and 7-celled at maturity
(ii) It is free-nuclear during the development
(iii) It is situated inside the integument but outside the nucellus
(iv) It has an egg apparatus situated at the chalazal end
(A) i and iv,
(B) ii and iii
(C) i and ii
(D) ii and iv
Answer:
(C) i and ii
Explanation :
Statement (i) and (ii) are correct regarding female gametophyte of flowering plant. The female gametophyte or embryo sac is located inside the nucellus, enclosed within the integuments. In a majority of flowering plants, one of the megaspores is functional while the other three degenerates. Three repeated mitotic divisions of the functional megaspore result in the formation of seven-celled or eight-nucleate embryo sac. Six of the eight nuclei are organized at the two poles. Three cells grouped at micropylar end form egg apparatus and 3 at the chalazal end form antipodal cells. The large central cell at the center has two polar nuclei. The meiotic divisions in the formation of embryo sac are strictly free nuclear, that is nuclear divisions are not followed immediately by cell-wall formation. Gametophyte is situated at micropylar end, not at chalazal end.

Chapter 2 Bio Class 12 MCQ Question 9.
Autogamy can occur in a chasmogamous flower if:
(A) Pollen matures before maturity of ovule
(B) Ovules mature before maturity of pollen
(C) Both pollen and ovules mature simultaneously
(D) Both anther and stigma are of equal lengths
Answer:
(C) Both pollen and ovules mature simultaneously
Explanation :
Autogamy is a method of self-pollination. It is a process in which the stigma of a flower receives pollen from the anther of same flower. For autogamy, both the sex organs of a chasmogamous flower should mature at the same time. As chasmogamous flowers open at maturity, pollen release and stigma receptivity should be synchronized for the process of autogamy. In such flowers, the length of anther and stigma plays a secondary role in autogamy, e.g., in case of protandry (in which pollens mature early) and protogyny (in which stigma matures early) leads to cross-pollination.
Reproduction In Flowering Plants Class 12 MCQ Question 10.
Choose the correct statement from the following:
(A) Cleistogamous flowers always exhibit autogamy
(B) Chasmogamous flowers always exhibit geitonogamy
(C) Cleistogamous flowers exhibit both autogamy and geitonogamy
(D) Chasmogamous flowers never exhibit autogamy
Answer:
(A) Cleistogamous flowers always exhibit autogamy
Explanation :
The pollination that occurs in opened flowers is called chasmogamy. It is of two types, that is, self-pollination (autogamy) and cross-pollination. Cross-pollination is of two types, that is, geitonogamy and xenogamy. So, we can say that chasmogamous flowers exhibit both autogamy (self-pollination) and allogamy (cross-pollination). While in cleistogamous flowers the anthers and stigma lie close to each other within the closed flowers. ‘ When anthers dehisce in [he flowers buds, pollen grains come in contact with the stigma or effective pollination. Thus, these flowers are invariably autogamous as there is no chance of cross-pollen landing on the stigma.
Class 12th Biology Chapter 2 MCQ Question 11.
A particular species of plant produces light, non-sticky pollen in large numbers and its stigmas are long and feathery. These modifications facilitate pollination by:
(A) Insects
(B) Water
(C) Wind
(D) Animals
Answer:
(C) Wind
Explanation :
Plants use two abiotic (wind and water) and one biotic (animals) agent to achieve pollination. Majority of plants use biotic agents for pollination. Pollination by wind is more common amongst abiotic pollination. It requires the light and non-sticky pollen grains so that, they can be transported in wind currents. They often possess well-exposed stamens (so that the pollens are easily dispersed into wind currents) and large often feathery stigma to easily trap air-borne pollen grains. Wind pollination is common in grasses. Pollination by water is called hydrophily which is quite rare in flowering plants but occurs in aquatic plants. Zoophily is pollination through the agency of animals. Entomophily (pollination by insects) is the most common type of zoophily which occurs through the agency of animals.
Biology Class 12 Chapter 2 MCQ Question 12.
From among the situations given below, choose the one that prevents both autogamy and geitonogamy
(A) Monoecious plant bearing unisexual flowers
(B)Dioecious plant bearing only male or female flowers
(C) Monoecious plant with bisexual flowers
(D) Dioecious plant with bisexual flowers
Answer:
Option (B) is correct
Explanation :
Dioecious plants (bearing only male or female flowers) prevent both autogamy and geitonogamy. Autogamy is a method of self-pollination in which the transfer of pollen grains from another to stigma of the same flower takes place. Geitonogamy is the transfer of pollen grains from another to stigma of another flower of the same plant. It is ecologically cross¬pollination which is supposed to be equivalent to self-pollination because all flowers on a plant are genetically identical.

MCQ Of Chapter 2 Biology Class 12 Question 13.
While planning for an artificial hybridization program involving dioecious plants, which of the following steps would not be relevant:
(A) Bagging of female flower
(B) Dusting of pollen on. stigma
(C) Emasculation
(D) Collection of pollen
Answer:
(C) Emasculation
Explanation :
Artificial hybridization is one of the major methods of crop improvement programs. This cross will make sure that only the desired pollen grains are used for pollination and the stigma is protected from contamination (from unwanted pollen). This is achieved by emasculation and bagging techniques. If the female parent produces unisexual flowers; there is no need for emasculation (a process of removal of anther). The female flower buds are bagged before the flowers open. When the stigma becomes receptive, pollination is carried out using the desired pollen and the flower rebagged. This protects them from contamination by unwanted pollen grains. When the female parent bears bisexual flowers, removal of anthers from the flower bud before the anther dehiscence is necessary,
Class 12 Biology Ch 2 MCQ Question 14.
In a flower, if the megaspore mother cell forms megaspores without undergoing meiosis and if one of the megaspores develops into an embryo sac, its nuclei would be:
(A) Haploid
(B) Diploid
(C) A few haploid and a few diploid
(D) With varying ploidy.
Answer:
(B) Diploid
Explanation :
In some species, the diploid egg cell is formed without reduction division and develops into an embryo without fertilization. IL is an asexual reproduction which occurs in the absence of pollinators or in extreme environments. In some species like citrus plants, nucellar cells surrounding ) the embryo sac start dividing and develop into embryos. It occurs in the megaspore mother cell without undergoing meiosis and produces diploid embryo sac through mitotic divisions. It helps in the preservation of desirable characters for indefinite period. Thus, it can be concluded that apomictic species produce diploid cells. Haploid cells will be formed during sexual reproduction when cells will undergo meiosis.
Class 12 Chapter 2 Biology MCQ Question 15.
Which one of the cell in an embryo-sac produces endosperm after double fertilization?
(A) Synergids cell
(B) Antipodal cell
(C) Central Cell
(D) Egg
Answer:
(C) Central Cell
Explanation :
In female gametophyte, central cell is involved in double fertilization that helps in the endosperm development While antipodal cells provide nourishment to the egg cell and synergid cell help in pollen tube growth.

Ch 2 Biology Class 12 MCQ Question 16.
Starting from the innermost part, the correct sequence of parts in an ovule are,
(A) egg, nucellus, embryo sac, integument
(B) egg, embryo sac, nucellus, integument
(C) embryo sac, nucellus, integument, egg
(D) egg, integument, embryo sac, nucellus.
Answer:
(B) egg, embryo sac, nucellus, integument
Explanation:
Starting from the innermost part, the correct sequence of parts in an ovule is egg, embryo-sac, nucellus, and integument.
MCQ Biology Class 12 Chapter 2 Question 17.
In a fertilized embryo sac, the haploid, diploid and triploid structures are:
(A) Synergid, zygote and primary endosperm nucleus
(B) Synergid, antipodal and polar nuclei
(C) Antipodal, synergid and primary endosperm nucleus
(D) Synergid, polar nuclei and zygote.
Answer:
(A) Synergid, zygote and primary endosperm nucleus
Explanation :
In a fertilized embryo sac, the haploid, diploid and triploid structures are synergids, zygote and primary endosperm nucleus respectively.
Class 12 Bio Chapter 2 MCQ Question 18.
In an embryo sac, the cells that degenerate after fertilization are:
(A) Synergids and primary endosperm cell
(B) Synergids and antipodals
(C) Antipodals and primary endosperm cell
(D) Egg and antipodals.
Answer:
(B) Synergids and antipodals
Explanation :
In unfertilized embryo sac, the antipodal sand synergids are distinctly present at chalazal end and micropylar end respectively while, in fertilized embryo sac, antipodals and synergids gradually degenerate after the formation of zygote.
Biology Chapter 2 MCQ Class 12 Question 19.
In the embryos of a typical dicot and a grass, true homologous structures are:
(A) Coleorhiza and coleoptile
(B) Coleoptile and scutellum
(C) Cotyledons and scutellum
(D) Hypocotyl and radicle.
Answer:
(C) Cotyledons and scutellum
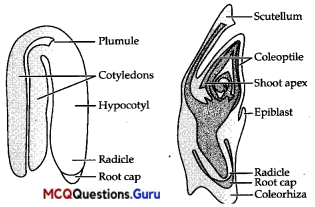
Explanation:
A typical dicotyledonous embryo consists of two cotyledons. While embryos of monocotyledons possess only one cotyledon and it is called scutellum (in grass). Cotyledons of dicots are simple structures generally thick and swollen due to storage of food reserves (as in legumes) and embryo of monocots consists of one large and shield-shaped cotyledon known as scutellum situated towards one side (lateral) of the embryonal axis.

MCQ Of Biology Class 12 Chapter 2 Question 20.
The phenomenon observed in some plants wherein parts of the sexual apparatus is used for forming embryos without fertilization is called:
(A) Parthenocarpy
(B) Apombds
(C) Vegetative propagation
(D) Sexual reproduction.
Answer:
(B) Apombds
Explanation :
Apomixis refers to the formation of seeds without fertilization. The embryos are genetically identical to the parental plan
Question 21.
The phenomenon wherein, the ovary develops into a fruit without fertilization is called:
(A) Parthenocarpy
(B) Apomixis
(C) Asexual reproduction
(D) Sexual reproduction
Answer:
(A) Parthenocarpy
Explanation :
Parthenocarpy is the formation of seedless fruits without fertilization. The fruits developed from unfertilized ovaries are ‘ called parthenocarpic fruits.
Question 22.
Fragrant flowers with well-developed nectaries are an adaptation for
(A)hydrophily
(B) anemophily
(C) entomophily
(D) none of these
Answer:
(C) entomophily
Explanation :
Entomophily is a type of pollination which occur by the insects, butterfly, wasp, ants, beetles and mainly by bees which is most common, the flowers are colourful attract the insect. Nectar is given as reward to insects.
Question 23.
The total number of nuclei involved in double fertilization in angiosperm are
(A) two
(B) three
(C) four
(D) five
Answer:
(D) five
Explanation:
Double fertilization is the process in angiosperms. It involves fusion of one male gamete (haploid) with egg (haploid) to form v zygote (diploid) that gives rise to embryo accompanied with fusion of another male gamete (haploid) with two polar nuclei (secondary nucleus) to form primary endosperm nucleus (PEN) that gives rise to a nutritive tissue called endosperm.
Question 24.
Heterostyly as a contrivance for cross-pollination is found in
(A) Pennisetum
(B) Impatiens
(C) Primula vulgaris
(D) Oenothera
Answer:
(C) Primula vulgaris
Explanation :
Heterostyly is the presence s of 2—3 types of flower with different heights of styles and stamens are dimorphic heterostyly, there are two types of flower, pin eyed (long style and short stamens) and thrum eyed (short style and long stamens), Primula vulgaris (primrose), jasmine.
Assertion and Reason Based MCQs
Directions: In the following questions a statement of assertion (A) is followed by a statement of reason (R). Mark the correct choice as :
(A) Both assertion (A) and Reason (R) are true and Reason (R) is the correct explanation of assertion (A).
(B) Both assertion (A) and Reason (R) are true but reason (R) is not the correct explanation of assertion (A).
(C) Assertion (A) is true but reason (R) is false.
(D) Assertion (A) is false but reason (R) is true.

Question 1.
Assertion (A): Tapetum is a part of another wall that has 2-3 layers of cells.
Reason (R): Tapetum layers help in development and growth of pollen grain.
Answer:
(B) Both assertion (A) and Reason (R) are true but reason (R) is not the correct explanation of assertion (A).
Explanation :
In flowering plants, tapetum are the specialized cells that provide nutrition to the pollen grain within the anther.
Question 2.
Assertion (A): Pollen grains are best preserved as fossils.
Reason (R): The sporopollenin of exine is highly resistant to the action of strong acids and alkali and can withstand a high temperature.
Answer:
(A) Both assertion (A) and Reason (R) are true and Reason (R) is the correct explanation of assertion (A).
Explanation:
Pollen grains are well preserved as fossils because of the presence of sporopollenin which is the most resistant organic material known.
Question 3.
Assertion (A): Tapetum is formed during the process of the formation of microsporangium.
Reason (R): The play an important role in guiding the pollen tubes into the synergid.
Answer:
(C) Assertion (A) is true but reason (R) is false.
Explanation :
Assertion is true, but the reason is wrong because tapetum plays an important role in nourishing pollen mother cells (PMCs)or microspores.
Question 4.
Assertion (A): Flowers are structure of sexual reproduction.
Reason (R): Different types of embryological process occur inside the flower.
Answer:
(A) Both assertion (A) and Reason (R) are true and Reason (R) is the correct explanation of assertion (A).
Explanation:
Sexual reproduction involves the transfer fusion of male and female gametes known as pollination. The fertilized ovules: produce seeds that continue the next generation.
Question 5.
Assertion (A): Cleistogamous flowers can produce seeds without pollination.
Reason (R): Cleistogamous flowers have no chance of cross-pollination and they are invariably autogamous.
Answer:
(A) Both assertion (A) and Reason (R) are true and Reason (R) is the correct explanation of assertion (A).

Question 6.
Assertion (A): Entomophilous flowers are large, colorful, fragrant and rich in nectar.
Reason (R): If helps in attracting the pollinating agent.
Answer:
(A) Both assertion (A) and Reason (R) are true and Reason (R) is the correct explanation of assertion (A).
Explanation :
Entomophily is a type of pollination, which is carried out by insects., Fragrance and color of the flowers attract insects.
Question 7.
Assertion (A): In Ophrys one petal of the flower bears on an uncanny resemblance to the female bee.
Reason (R): Two closely related species competing for the same resource can co-exist simultaneously.
Answer:
(A) Both assertion (A) and Reason (R) are true and Reason (R) is the correct explanation of assertion (A).
Question 8.
Assertion (A): Perisperm is a haploid tissue.
Reason (R): Perisperm is the remains of nucellus which surround the embryo in certain seeds.
Answer:
(D) Assertion (A) is false but reason (R) is true.
Explanation:
Perisperm is a nutritive tissue of a seed derived from the nucellus and deposited | externally to the embryo sac. It is diploid.
Question 9.
Assertion (A): Pea, bean, mustard are non- albuminous seeds.
Reason (R): These seeds retain a part of endosperm as it is not completely used up during embryo development.
Answer:
(C) Assertion (A) is true but reason (R) is false.
Explanation :
Assertion is true but the reason is wrong because, in non-albuminous seeds, seeds does not retain any endosperm as it is completely used up during embryo development.
Question 10.
Assertion (A): Geitonogamous flowering plants are cross-pollinated plants.
Reason (R): In geitonogamous flowering plants the pollen is transferred to the stigma of another flower of another plant.
Answer:
(C) Assertion (A) is true but reason (R) is false.
Explanation:
A is true but R is wrong because in geitonogamy flower the pollen is transferred to the stigma of another flower of the same plant.
Question 11.
Assertion (A): Fertilization in flowers, produces fruits and seeds.
Reason (R): After fertilization, the ovary develops into fruits and ovule develops into seed.
Answer:
(A) Both assertion (A) and Reason (R) are true and Reason (R) is the correct explanation of assertion (A).
Question 12.
Assertion (A): Seed is the final product of sexual reproduction in angiosperms.
Reason (R): A seed typically bears seed coat, cotyledons and an embryo axis.
Answer:
(B) Both assertion (A) and Reason (R) are true but reason (R) is not the correct explanation of assertion (A).
Explanation :
After fertilization, the ovary wall develops in fruits embryos captured in the seed as final product of sexual reproduction in plants. The seed bear protective seed coat, cotyledons, and embryo axis.

Case-Based MCQs
Attempt any 4 sub-parts from each question. Each sub-part carries 1 mark.
I. Read the following text and answer the following questions on the basis of the same:
Study the given diagram and answer any of the four questions given below:
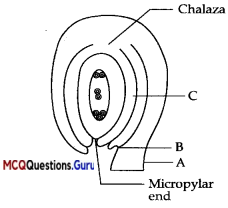
Question1.
This diagram represents which type of ovule
(A) Atropous
(B) Orthotropous
(C) Anatropous
(D) Amphitropous
Answer:
(C) Anatropous
Explanation:
Anatropous ovule is a completely inverted ovule turned back 180 degrees on its stalk. Ovule – a small body that contains the female germ cell of a plant; develops into a seed after fertilization.
Question 2.
A is the stalk of the ovule is called
(A) Hilum
(B) Pedicle
(C) Chalazal pole
(D) Funicle
Answer:
(D) Funicle
Explanation :
Funicle is Ihi’ stalk that attaches an ovule to the placenta in the ovary of a flowering plant. It contains a strand of conducting tissue leading from the placenta | into the chalaza.
Question 3.
The junction of attachment of funicle with the body of ovule at B is
(A) Funicle
(B) Hilum
(C) Nucellus
(D) Chalazal pole
Answer:
(B) Hilum
Explanation :
A scar on a seed (as a bean) marking the point of attachment of the ovule is called hilum. Ihere is small pore, called micropyle, which represent the micropyle of ovule.

Question 4.
Tegmen develops from the part labelled C in the figure is called
(A) Inner integument
(B) Outer Integument
(C) Funicle
(D) Chalazal pole
Answer:
(A) Inner integument
Explanation :
The seed has two layers one is outer called the testa which is developed from the outer integument and another is inner layer called the legmen which is develops from the inner integument of the ovule.
Direction: In the following questions a statement of Assertion (A) is followed by a statement of Reason (R). Mark the correct choice as :
(A) Both assertion (A) and reason (R) are true and (R) is correct explanation of assertion (A).
(B) Both assertion (A) and reason (R) are true, but reason (R) is not the correct explanation of assertion (A).
(C) Assertion (A) is true, but reason (R) is false.
(D) Assertion (A) is false, but reason (R) is true.
Question 5.
Assertion (A): Most common type of ovule is anatrous.
Reason (R): Anatropous ovule is horse-shoe shaped.
Answer:
(C) Assertion (A) is true, but reason (R) is false.
Explanation :
Funiculus lies at the micropylar end and due to the unilateral growth of the ovule is called anatropous ovule. In angiosperms, when the curvature of the ovule affects the nucellus and later it becomes horseshoe-shaped. Such ovule is called amphitropous.
II. Read the following text and answer the following questions on the basis of the same:
Gynoecium is the female reproductive part of the flower. It may consist of a single or more than one pistil. This pistil may be free or fuse. Each pistils has three parts, stigma, style and ovary. Ovary has an ovarian cavity, which has one or many chambers or locules. The placenta is located inside the ovarian cavity. Megasporangia or ovules arise from the placenta.
Question 1.
In which of the following plants the number of ovules in an ovary is one?
(A) Mango
(B) Orchids
(C) Watermelon
(D) Papaya
Answer:
(A) Mango
Explanation :
Mango possess a single ovule in each ovary and orchids, watermelon, and papaya have multiple ovules present in each ovary.
Question 2.
A multicapellary, syncarpous gynoecium is found in:
(A) Papaver
(B) Brinjal
(C) Tomato
(D) All
Answer:
(D) All
Explanation :
Papaver, brinjal, and tomato all have multicar Bellary, syncarpous gynoecium. In this condition, carpels are more than one and fused.

Question 3.
82% of ovules found in angiosperms are
(A) Anatropous
(B) Amphitropous
(C) Orthotropous
(D) Circinotropous
Answer:
(A) Anatropous
Explanation:
anatropous ovule is found in 82% angiosperm and it completely inverted ovule turned back 180 degrees on its stalk.
Question 4.
Which among the following cell is binucleate in an embryo sac?
(A) Antipodal cell
(B) Central cell
(C) Synergid
(D) Female gamete
Answer:
(B) Central cell
Explanation :
Central cell form binucleate endosperm mother cell upon fertilization with one of the two sperm cells, forms triploid endosperm to nourish embryo development.
Question 5.
Flowers with both androecium and gynoecium are called:
(A) Bisexual flowers
(B) Anther
(C) Unisexual flowers
(D) Androgynous
Answer:
(B) Anther
Explanation :
Androecium is the male part and gynoecium is the female part, and in those flowers have both of these they are called bisexual flowers.
III. Read the following text and answer the following questions on the basis of the same:
A typical anther is bilobed. It is a tetragonal structure consisting of four microsporangia. These microsporangia form pollen sac which on maturity
gets filled with a pollen grains. Pollen grains represent the male gametophytes, their cell wall is very hard. Pollen grains of many species cause severe allergies which cause various diseases in human beings.
Question 1.
Which among the following is a major cause of pollen allergy in India?
(A) Mirabilis
(B) Myosotis
(C) Parthenium
(D) Pistia
Answer:
(C) Parthenium
Explanation :
Parthenium is an invasive species in India, and its is also known as carrot grass or congress grass which is the major cause of allergy in India, the parthenium weed produces as much as 3,000 million pollen grains per square meter during the flowering season.
Question 2.
Select the odd one out with respect to wall layers of microsporangium in flowering plants.
(A) Integument
(B) Tapetum
(C) Endothecium
(D) Middle layers
Answer:
(A) Integument
Explanation :
The integuments arc the outer layer(s) of the ovule and develop into a seed 3 coat as the ovule matures following fertilisation.

Question 3.
Study of pollen grains is called
(A) Bryology
(B) Mycology
(C) Algology
(D) Polynology
Answer:
(D) Polynology
Explanation :
Study of pollen grains is called polynology.
Question 4.
The prominent pollen grain aperture called germ pore is present in :
(A) Exine
(B) Intine
(C) Vegetative ceU
(D) Generative ceU
Answer:
(A) Exine
Explanation :
The prominent pollen grain aperture called germ pore is present in exine, it is decay-resistant outer coating of a pollen grain or spore.
Direction: In the following questions a statement of Assertion (A) is followed by a statement of Reason (R). Mark the correct choice as :
(A) Both assertion (A) and Reason (R) are true and (R) is correct explanation of assertion (A).
(B) Both assertion (A) and Reason (R) are true, but reason (R) is not the correct explanation of assertion (A).
(C) Assertion (A) is true, but reason (R) is false.
(D) Assertion (A) is false, but reason (R) is true.

Question 5.
Assertion (A): The innermost layer of microsporangium is called tapetum.
Reason (R): Tapetum nourishes the development into pollen grains.
Answer:
(B) Both assertion (A) and Reason (R) are true, but reason (R) is not the correct explanation of assertion (A).
Explanation :
Cells of tapetum have dense cytoplasm and more than one nuclei, which help in nourishing the developing pollen grains.