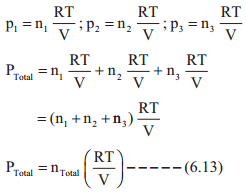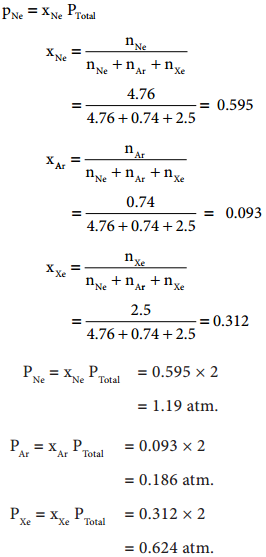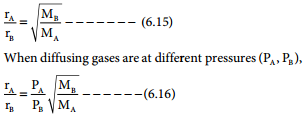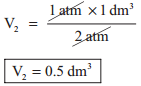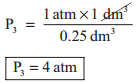Find free online Chemistry Topics covering a broad range of concepts from research institutes around the world.
The Gas Laws
The gas laws have played a major role in the development of chemistry. The physical properties of all gases are governed by the gas laws that were formulated based on the studies of the properties like pressure, volume, etc., as a function of temperature. Before studying the gas laws in detail, let us understand an important parameter, namely, the pressure.
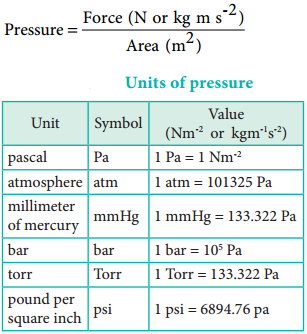
Pressure is defined as force divided by the area to which the force is applied. The SI unit of pressure is pascal which is defined as 1 Newton per square meter (Nm-2). There are other units that are commonly used and their relation with the SI unit is as follows.
Boyle’s Law: Pressure-Volume
Relationship
Robert Boyle performed a series of experiments to study the relation between the pressure and volume of gases. The schematic of the apparatus used by him is shown in figure 6.1.
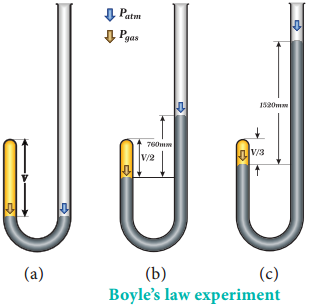
Mercury was added through the open end of the apparatus such that the mercury level on both ends are equal as shown in the figure 6.1 (a). Add more amount of mercury until the volume of the trapped air is reduced to half of its original volume as shown in figure 6.1(b). The pressure exerted on the gas by the addition of excess mercury is given by the difference in mercury levels of the tube.
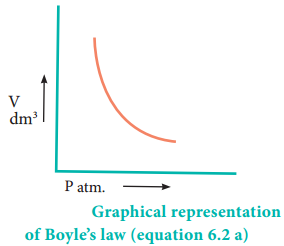
Initially the pressure exerted by the gas is equal to 1 atm as the difference in height of the mercury levels is zero. When the volume is reduced to half, the difference in mercury levels increases to 760 mm. Now the pressure exerted by the gas is equal to 2 atm. It led him to conclude that at a given temperature the volume occupied by a fixed mass of a gas is inversely proportional to its pressure.
Mathematically, the Boyle’s law can be written as
V α \(\frac{1}{P}\) ……………… (6.1)
(T and n are fixed, T-temperature, n- number of moles)
V = k × \(\frac{1}{P}\) ……………… (6.2)
k – proportionality constant
When we rearrange equation 6.2.
PV = k ………. (6.2a) (at constant temperature and mass)
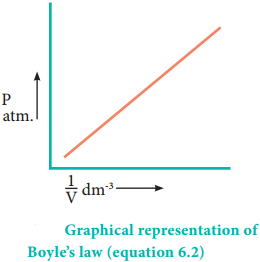
Boyle’s law is applicable to all gases regardless of their chemical identity (provided the pressure is low). Therefore, for a given mass of a gas under two different sets of conditions at constant temperature we can write
P1V1 = P2V2 = K ………… (6.3)
P1V1 = P2V2 = k …………. (6.3)
The PV relationship can be understood as follows. The pressure is due to the force of the gas particles on the walls of the container. If a given amount of gas is compressed to half of its volume, the density is doubled and the number of particles hitting the unit area of the container will be doubled. Hence, the pressure would increase two fold.
Consequence of Boyle’s Law
The pressure-density relationship can be derived from the Boyle’s law as shown below.
P1V1 = P2V2 (Boyle’s law)
P1\(\frac{m}{d1}\) = P2\(\frac{m}{d2}\)
where “m” is the mass, d1 and d2 are the densities of gases at pressure P1 and P2 ………… (6.4)
In other words, the density of a gas is directly proportional to pressure.
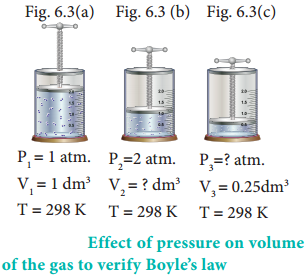
In figure (6.3) let us find the missing parameters (volume in 6.3 (b) and pressure in 6.3 (c))
Solution:
According to Boyle’s law, at constant temperature for a given mass of gas at constant temperature,
P1V1 = P2V2 = P3V3
1 atm × 1 dm3 = 2 atm × V2 = P3 × 0.25 dm3
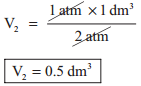
and P3 × 0.25 dm3 = 1 atm × 1 dm3
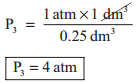
Charles Law (Volume-temperature relationship)
The relationship between volume of a gas and its temperature was examined by J. A. C. Charles. He observed that for a fixed mass of a gas at constant pressure, the volume is directly proportional to its temperature (K). Mathematically it can be represented as (at constant P and n)
V = kT ………….. (6.5)
or \(\frac{V}{T}\) = Constant
If the temperature of the gas increases, the volume also increases in direct proportion, so that \(\frac{V}{T}\) is a constant. For the same system at constant pressure, one can write
\(\frac{\mathrm{V}_{1}}{\mathrm{~T}_{1}}=\frac{\mathrm{V}_{2}}{\mathrm{~T}_{2}}\) = Constant ………….. (6.6)
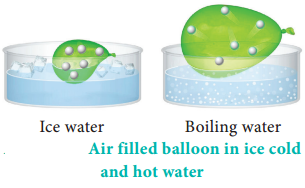
For example, if a balloon is moved from an ice cold water bath to a boiling water bath, the temperature of the gas increases. As a result, the gas molecules inside the balloon move faster and gas expands. Hence, the volume increases.
Variation of Volume with Temperature at Constant Pressure
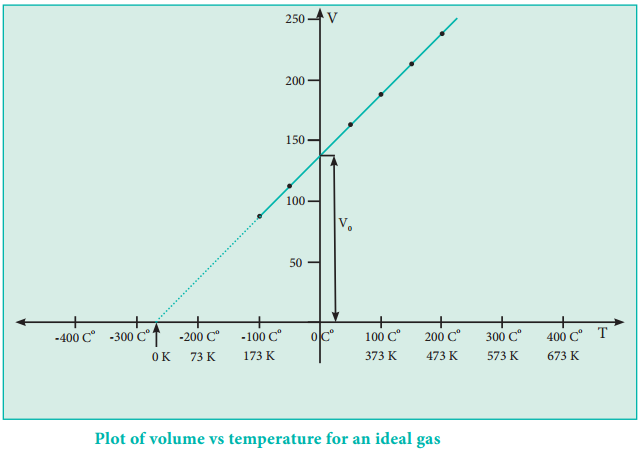
The plot of the volume of the gas against its temperature at a given pressure is shown in the figure 6.5. From the graph it is clear that the volume of the gas linearly increases with temperature at a given pressure. Such lines are called isobars. It can be expressed by the following straight line equation.
V = mT + C where T is the temperature in degree Celsius and m & C are constants.
When T = 0ºC the volume becomes V0 = C and slope of the straight line m is equal to ΔV/ΔT. Therefore the above equation can be written in the following form.
V = (\(\frac{∆V}{∆T}\))T + V0 …………… (6.7) (n, P are constant)
Divide the equation 6.7 by V0
Charles and Gay Lussac found that under constant pressure, the relative increase in volume per degree increase in temperature is same for all gases. The relative increase in volume per ºC (α) is equal to \(\frac{1}{V_{0}}\)(\(\frac{∆V}{∆T}\)).
Therefore
\(\frac{V}{V_{0}}\) = αT + 1
V = V0(αT + 1) ………. (6.9)
Charles found that the coefficient of expansion is approximately equal to 1/273. It means that at constant pressure for a given mass, for each degree rise in temperature, all gases expand by 1/273 of their volume at 0ºC
If we extrapolate the straight line in the figure 6.5 beyond the experimental measurements, the straight line intersects the temperature axis (x-axis) at -273º C. This shows that the volume of the gas becomes zero at -273º C, more precisely this temperature is -273.15º C. Beyond this temperature the gas would have a negative volume which is physically impossible.
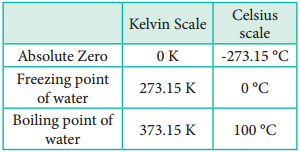
For this reason, this temperature was defined as absolute zero by Kelvin and he proposed a new temperature scale with absolute zero as starting point which is now called Kelvin scale. The only difference between the Kelvin scale of temperature and Celsius scale of temperature is that the zero position is shifted. The boiling and freezing point of water in both scales are given below.
Example:
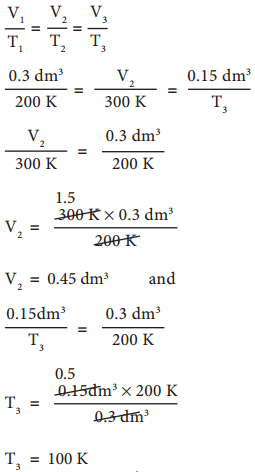
In figure 6.6 let us find the missing parameters (volume in 6.6 (b) and temperature in 6.6(c))
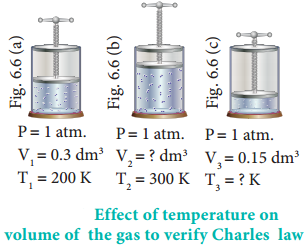
Solution:
According to Charles law,
Gay-Lussac’s Law (Pressure Temperature Relationship)
Joseph Gay-Lussac stated that, at constant volume the pressure of a fixed mass of a gas is directly proportional to temperature. or \(\frac{P}{T}\) = Constant k
If P1 and P2 are the pressure at temperatures T1 and T2, respectively, then
from Gay Lussac’s law
\(\frac{P_{1}}{T_{1}}=\frac{P_{2}}{T_{2}}\)
Avogadro’s Hypothesis
Avogadro hypothesised that equal volumes of all gases under the same conditions of temperature and pressure contain equal number of molecules. The mathematical form of Avogadro’s hypothesis may be expressed as
V α n,
\(\frac{V_{1}}{n_{1}}=\frac{V_{2}}{n_{2}}\) = Constant ……….. (6.10)
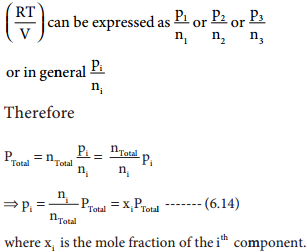
where V1 & n1 are the volume and number of moles of a gas and V2 & n2 are a different set of values of volume and number of moles of the same gas at same temperature and pressure.