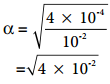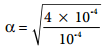Find free online Chemistry Topics covering a broad range of concepts from research institutes around the world.
Common ion Effect
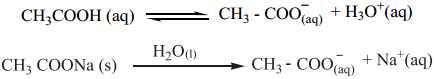
When a salt of a weak acid is added to the acid itself, the dissociation of the weak acid is suppressed further. For example, the addition of sodium acetate to acetic acid solution leads to the suppression in the dissociation of acetic acid which is already weakly dissociated. In this case, CH3COOH and CH3COONa have the common ion, CH3COO–.
Let us analyse why this happens. Acetic acid is a weak acid. It is not completely dissociated in aqueous solution and hence the following equilibrium exists. CH3COOH(aq) ⇄ H+(aq) + CH3COO–(aq).
However, the added salt, sodium acetate, completely dissociates to produce Na+ and CH3COO– ion.
CH3COONa(aq) → Na+(aq) + CH3COO–(aq)
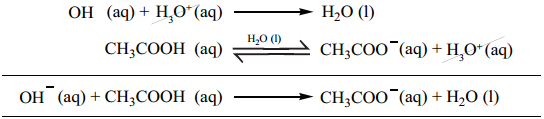
Hence, the overall concentration of CH3COO– is increased, and the acid dissociation equilibrium is disturbed. We know from Le chatelier’s principle that when a stress is applied to a system at equilibrium, the system adjusts itself to nullify the effect produced by that stress.
So, in order to maintain the equilibrium, the excess CH3COO– ions combines with H+ ions to produce much more unionized CH3COOH i.e, the equilibrium will shift towards the left. In other words, the dissociation of a weak acid (CH3COOH) is suppressed. Thus, the dissociation of a weak acid (CH3COOH) is suppressed in the presence of a salt (CH3COONa) containing an ion common to the weak electrolyte. It is called the common ion effect.
The common-ion effect refers to the decrease in solubility of an ionic precipitate by the addition to the solution of a soluble compound with an ion in common with the precipitate. This behaviour is a consequence of Le Chatelier’s principle for the equilibrium reaction of the ionic association/dissociation.
The common ion effect is the phenomenon in which the addition of an ion common to two solutes causes precipitation or reduces ionization. An example of the common ion effect is when sodium chloride (NaCl) is added to a solution of HCl and water.
The common ion effect suppresses the ionization of a weak acid by adding more of an ion that is a product of this equilibrium. The common ion effect suppresses the ionization of a weak base by adding more of an ion that is a product of this equilibrium. The reaction is put out of balance, or equilibrium.
Aquifers which contain chalk and limestone, a common ion effect is used in these to obtain drinking water. Calcium carbonate is sparingly soluble in water, and it can be precipitated out by adding sodium chloride in the solution. In this way, the common ion effect is used in treatment of water.
According to Le Chatelier’s principle, addition of more ions alters the equilibrium and shifts the reaction to favor the solid or deionized form. In the case of an an acidic buffer, the hydrogen ion concentration decreases, and the resulting solution is less acidic than a solution containing the pure weak acid.
Le Chatelier’s principle is an observation about chemical equilibria of reactions. It states that changes in the temperature, pressure, volume, or concentration of a system will result in predictable and opposing changes in the system in order to achieve a new equilibrium state.
At a given temperature, the product of water concentrations and ions is known as the ionic product of water. With the rise in temperature, the value increases i.e. the concentration of H+ and OH– ions increases with temperature increases.
If you have a solution and solute in equilibrium, adding a common ion (an ion that is common with the dissolving solid) decreases the solubility of the solute. This is because Le Chatelier’s principle states the reaction will shift toward the left (toward the reactants) to relieve the stress of the excess product.
pH gives us the measure of acid/base strength of any solution. pH scale ranges from 0-14, 7 is neutral, below 7 it represents acidic nature i.e. the compound is acidic and above 7 it represents basic nature i.e. the compound is basic i.e. pH is the negative logarithm of hydrogen ion.
The solubility of slightly soluble substances can be decreased by the presence of a common ion. This effect is known as common ion effect. Addition of a common ion to a slightly soluble salt solution will add up to the concentration of the common ion.
Le Chatelier’s principle (also known as “Chatelier’s principle” or “The Equilibrium Law”) states that when a system experiences a disturbance (such as concentration, temperature, or pressure changes), it will respond to restore a new equilibrium state.
Explanation:
This phenomenon is called the common-ion effect. When a compound dissolves in water it dissociates into ions. Increasing the concentration of one of these ions will shift the equilibrium towards the compound, thereby making it hard for the compound to dissolve in water (decreases solubility of compound).
When there is an increase in pressure, the equilibrium will shift towards the side of the reaction with fewer moles of gas. When there is a decrease in pressure, the equilibrium will shift towards the side of the reaction with more moles of gas.
The classic example of the practical use of the Le Chatelier principle is the Haber-Bosch process for the synthesis of ammonia, in which a balance between low temperature and high pressure must be found.
Le Chatelier’s Principle helps to predict what effect a change in temperature, concentration or pressure will have on the position of the equilibrium in a chemical reaction. This is very important, particularly in industrial applications, where yields must be accurately predicted and maximised.
![]()
![]()
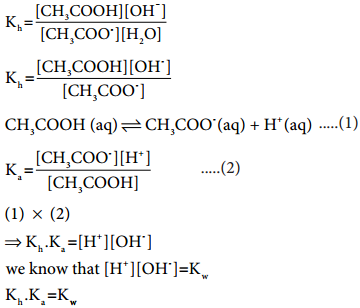
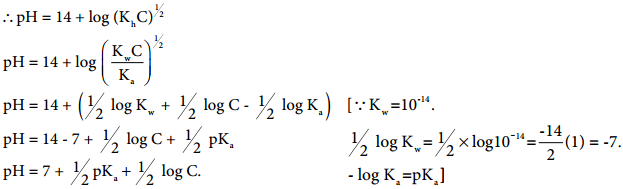
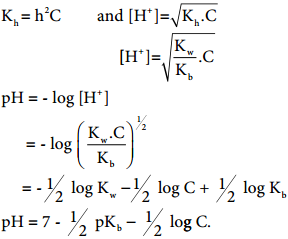
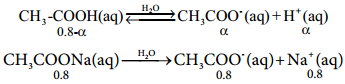
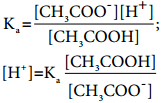
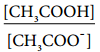
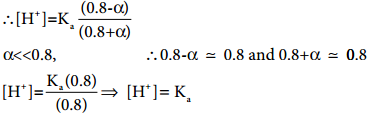
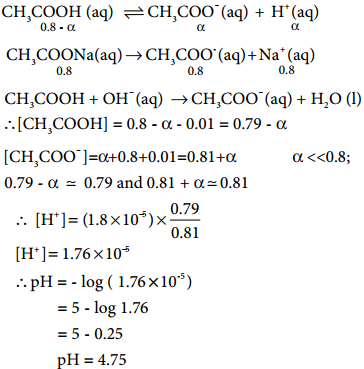
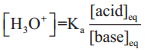 ………….. (8.20)
………….. (8.20)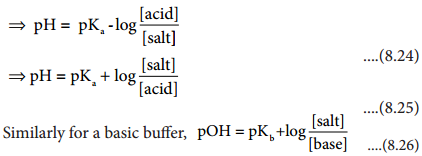
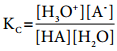 …………. (8.9)
…………. (8.9)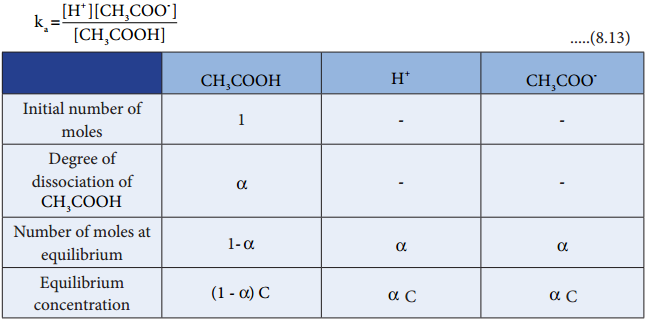
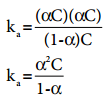 …………. (8.14)
…………. (8.14)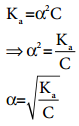 ………….. (8.15)
………….. (8.15)