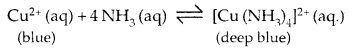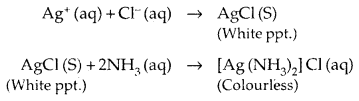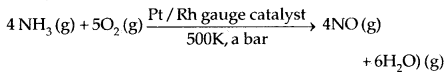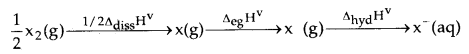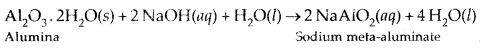By going through these CBSE Class 12 Chemistry Notes Chapter 8 The d-and f-Block Elements, students can recall all the concepts quickly.
The d-and f-Block Elements Notes Class 12 Chemistry Chapter 8
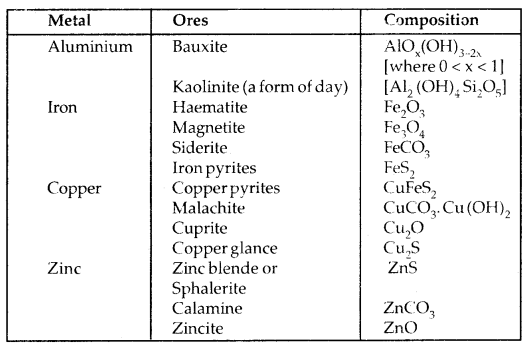
Transition Elements: d-Block. elements are called transition elements. They are placed in between the s-block on their left and the p-block on their right. They are called transition elements because they have properties intermediate between those of s- and p-block elements and represent a change from the most electropositive s-block elements to the least electropositive elements.
This element has partly filled (n -1) d-subshell in their atomic or ionic state. They are called d-block elements since in them 3d, 4d, 5d and 6d subshells are incomplete and the last electron enters (n -1) d subshell, i.e., penultimate (last but one) shell.
General Electronic Configuration:
The outermost shell: Their general electronic configuration is (n – 1) d1-10 ns0-2 where n is the outermost shell. Filling of 3d, 4d, 5d and 6d subshells leads to first transition series [4th period], 2nd transition series [5th period], 3rd transition series [6th period] and 4th incomplete transition series [7th period] respectively.
1st Transition Series or 3d series: Starts from Sc (At. No. 21) and ends at Zinc (Zn; atomic no. = 30).
Their configuration is 3d1-10 ns1-2.
- Scandium (Sc) = 21 = [Ar]18 3d1 4s2.
- Titanium (Ti) = 22 = [Ar]18 3d2 4s2
- Vanadium (V). = 23 = [Ar]18 3d3 4s2
- Chromium (Cr) = 24 = [ Ar]18 3d5 4s1
- Manganese (Mn) = 25 = [Ar]18 3d6 4s2
- Iron (Fe) = 26 = [Ar]18 3d6 4s2
- Cobalt (Co) = 27 = [Ar]18 3d7 4s2
- Nickel (Ni) = 28 = [Ar]18 3d8 4s2
- Copper (Cu) = 29 = [Ar]18 3d10 4s1
- Zinc (Zn) == 30 = [Ar]18 3d10 4s2
It may be noted that the electronic configuration of chromium and copper given above are exceptional. They have a single electron in the 4s-orbital. It is due to the extra-stability of 3d5 and 3d10 (half-filled and completely filled) orbitals. They consist up of 10 elements.
2nd Transition Series or 4d Series: Starts from the element Ytterium (Y) [Z = 39] and ends of cadmium (Cd; Z = 46). Their electronic configuration is 4d0-10 5s1-2. Palladium (Pd; Z = 46) has exceptional configuration of 4d10 5s°. They consists up of ten elements.

Third Transition Series or 5d series: Corresponds to filling of 5d sub-level. They consist up of ten elements La (Z = 57), H/(Z = 72), Ta, W, Re, Os, Ir, Pt, Au and Hg(Z = 80).
The electronic configuration of 3rd transition elements in the inner and valence shell is given below:

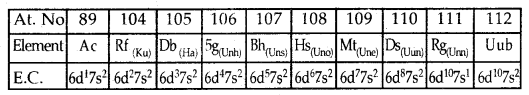
Fourth Transition Series or 6d Series: Corresponds to tire filling up of 6d Sub level and starts with Actinium (Ac; Z = 89), Rf (earlier Ku, Z = 104), Db, Sg, Bh, Hs, Mt, Ds, Rg and ends at Uub (Z = 112).
The fundamental difference in the electronic configuration of Representative Elements and Transition Elements: In the representative elements (s- and p-block elements), the valence electrons are present only in the outermost shell while in the transition elements, the valence electrons are present in the outermost shell as well as d- orbitals of the penultimate shell.
Zinc (Zn), Cadmium (Cd) and mercury (Hg) are misfits according to the definition of transition elements as they have 3d10, 4d10, 5d10 (completely filled inner d-orbitals) in the ground state of their atoms or in one of the common oxidation states (dipositive ions: Zn2+, Cd2+, Hg2+) respectively. They do not show properties of transition elements to any appreciable extent, except for their ability to form complexes.
The d-block of the periodic table contains the elements of Groups 3-12 in which the d-orbitals progressively filled in each of the four long periods.
General Properties of Transition Elements:
(a) Metallic character: All the elements of d block are metals. This is due to the fact that they have low ionisation energy values and have only one dr two s-electrons in the outermost shell. The transition elements exhibit good mechanical properties i.e., they are hard, malleable and ductile. Except for Zn, Cd and Hg, they have high m. and b.pts. They have thermal and electrical conductivity and metallic lustre.
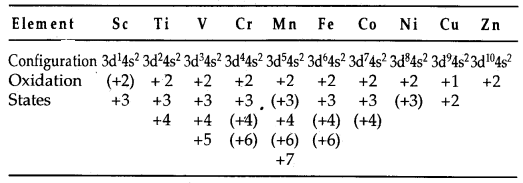
(b) Variable oxidation states: The transition elements exhibit a variety of oxidation states in their compounds. This is due to the fact that (n -1) d orbitals are of comparable energy to ns orbitals and therefore some or all of the (n -1) d electrons can be used along with ns electrons in compounds formation. Some common oxidation states exhibited by elements of the first transition series are listed below:
(The value in parentheses are less common oxidation states.)
(c) Coloured compounds: Most of the compounds of transition elements are coloured. The d orbitals in transition metal compounds are not of equal energy. In transition elements, the d-orbital are partly filled and electron may be promoted from one d level to another d level by absorbing visible light. Consequently, the compound has the colour complement to the absorbed light. For example, Cu2 absorbs red light and transmitted light contains an excess of the other colours of the spectrum and appears to be blue.
The ions Zn2+, Cu+, Ti4+, Sc3+ are white because they have either completely empty or completely filled d-subshell.
(d) Magnetic properties: Most of the transition metals and their compounds are paramagnetic i.e., they are attracted by the magnetic field. This, the property is due to the presence of unpaired electrons in the d orbitals of their atoms. The elements like iron, nickel and cobalt have appreciable paramagnetism and are called ferromagnetic substances. The magnetic moment of compounds of d-block elements are determined by spin only values which may be related to the number of impaired electrons (n) as
Spin magnetic moment (μs) = \(\sqrt{n(n+2)}\)
The agreement between the observed and calculated values is quite good for most of the elements. However, for the latter half of the series, the contribution from orbital magnetic moments is observed.
(e) Complex formation: The transition elements have a great tendency to form complexes.
This is because of:
- their small cation size
- high effective nuclear charge and
- presence of empty d-orbitals of appropriate energy for bonding to the ligands.
It has been noticed that
- In each transition series, the stability of complexes increases with an increasing atomic number of the element and in a particular oxidation state with decreasing size of its atoms.
- In the case of metal atoms that show more than one oxidation state, the complexes of greater stability are formed with the metal ions of the highest charge.
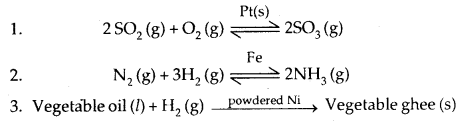
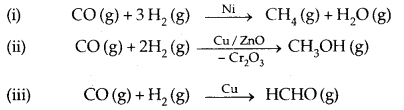
(f) Catalytic properties: Many transition metals and their compounds show catalytic properties e.g., Fe, Pt, V2O5, Ni etc. This property may be due to either the use of the d-orbitals or from the formation of transient intermediate compounds to absorb and activate the reacting substances.
(g) Alloy Formation: Transition metals mix freely with each other in the molten state and on cooling a solution of different transition metals to form alloys. For example, chromium dissolves in nickel to form Cr-Ni alloy; manganese dissolves in iron to form manganese steel. They take part in alloy formation because their atoms can exchange lattice sites of each other. Such alloys are formed by atoms with metallic radii that are within about 15 per cent of each other.
(h) Formation of Interstitial Compounds: Interstitial compounds are those which are formed when small atoms like H, C or N are trapped inside the crystal lattices of metals. They are usually non-stoichiometric and are neither typically ionic nor covalent. Many of the transition metals form interstitial compounds particularly with small non-metal atoms such as hydrogen, boron, carbon and nitrogen.
These small atoms enter into the voids sites between the packed atoms of the crystalline metal, e.g., TiC, Mn4N, Fe3M and TiH2 etc. The formulae quoted do not, of course, correspond to any normal oxidation state of the metal and often non-stoichiometric material is obtained with such composition as VH056 and TiH17. Because of the nature of their composition, these compounds are referred to as interstitial compounds.
The principal physical and chemical characteristics of these compounds are as follows:
- They have high melting points, higher than those of pure metals.
- They are very hard, some borides approach diamond in hardness.
- They retain metallic conductivity.
- They are chemically inert.
Some Important Compounds of Transition Metals:
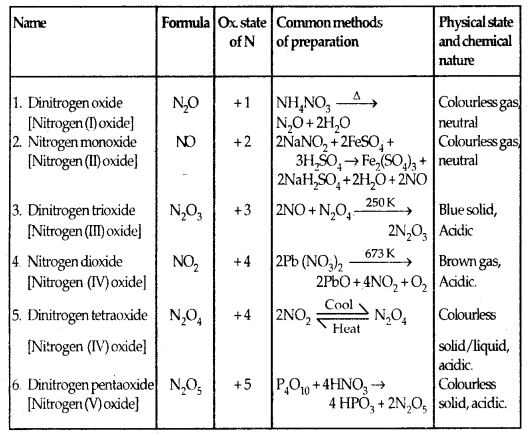
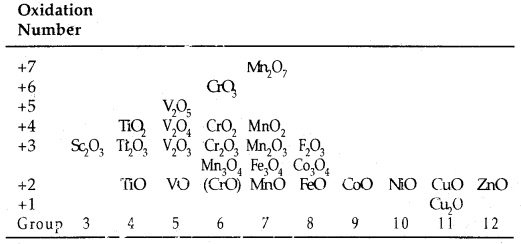
Oxides: Transition metal oxides are formed by the action of oxygen with transition metals at high temperature. The general formulas of the oxides of transition metals are MO, M2O3, M3O4, MO2, M2O5 and MO3.
It has been observed that:
- the oxides in which the metals are in a lower oxidation state are basic in nature.
- the oxides in which the metal is in a higher oxidation state are acidic in nature.
- the oxides in which the metal exhibits intermediate oxidation states are amphoteric.
For example, among the oxides of manganese, MnO is basic, Mn2O3, Mn3O4 and MnO2 are amphoteric while Mn2O7 is acidic.
Oxides Of 3d Metals:
Chemical Reactivity and E° Values: Transition metals vary widely in their chemical reactivity. Many of them are sufficiently electropositive to dissolve in mineral acids, although a few are ‘noble’: that is, they are unaffected by simple acids.
The metals of the first series with the exception of copper are relatively more reactive and are oxidised by 1M H+, though the actual rate at which these metals react with oxidising agents like hydrogen ion (H+) is sometimes slow. For example, titanium and vanadium, in practice, are passive to dilute non-oxidising acids at room temperature. The Ee values for M2+/M indicate a decreasing tendency to form divalent cations across the series.
This general trend towards less negative Ee values is related to the increase in the sum of the first and second ionisation enthalpies. It is interesting to note that the Ee values for Mn, Ni, and Zn are more negative than expected from the general trend. Whereas the stabilities of half-filled d subshell (d5) in Mn2+ and completely filled d subshell (d10) in zinc are related to their Eθ values; for nickel, Eθ value is related to the highest negative enthalpy of hydration.
An examination of the Eθ values for the redox couple M3+/M2+ shows that Mn3+ and CO3+ ions are the strongest oxidising agents in aqueous solutions. The ions Ti2+, V2+ and Cr2+ are strong reducing agents and will liberate hydrogen from a dilute acid. e.g.
2 Cr2+(aq) + 2 H+(aq) → 2 Cr3+(aq) + H2(g)
Trends in Stability of Higher oxidation states as exhibited in the oxides and halides of 3d-Series:
The table below shows the stable halides of the 3d series of transition metals. The highest oxidation numbers are achieved in Ti tetrahalides, VF5 and CrF6. The +7 state for Mn is not represented but MnOF3 is known and beyond Mn, no metal has a trihalide except Fe and COF3.
The ability of F to stabilize the higher oxidation state is due to either higher lattice energy of the higher compound as in the case of COF3 or higher bond energy terms for the higher covalent compounds e.g. VF5 and CrF6.
Table: Formulae of halides of groups 4 to 12:
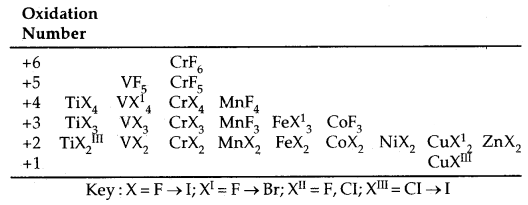
Halides: Transition metals react with halogens at high temperature to form transition metal halides. The order of reactivity of halogens is F > Cl > Br > I. The halides of higher oxidation states are the fluorides because metals can be oxidised to higher oxidation states with only fluorine which is most reactive. Bonding in fluorides is mainly ionic. In chlorides, bromides and iodides, the ionic character decrease with the atomic mass of halogen.
The ability of O to stabilize the higher oxidation state is also demonstrated in the oxohalides. Although Vv is represented only by VF5, the oxohalides VOX3 are known where X = F, Cl or Br. Another feature of fluorides is their instability in the low oxidation states e.g., VX2 (X = Cl, Br or I) and the same applies to CuX. On the other hand, all Cu11 halides are known except the iodide, in this case, Cu2+ oxidises I– to I2:
Cu2+ + 2I–→ CuI(S) + \(\frac{1}{2}\) I2
However Cul is unstable in solution and undergoes disproportionation.
2Cu+ → Cu2+ + Cu
The stability of Cu2+ (aq) rather than Cu+ (aq) is due to the much more negative Ah d H ° of Cu2+ (aq) than Cu+ (aq), which more than compensates for the 2nd IE of Cu.
The highest oxidation number in the oxides (Table) coincides with the group number and is attained in Sc2O3, to Mn2O7. Beyond Group 7, no higher oxides of Fe above Fe2O3, are known, although ferrates (VI):(FeO4)2- are formed in alkaline media they readily decompose to Fe2O3 and O2. Besides the oxides, oxidations stabilise Vv as VO2+, Vlv VO2+ and TiIV as TiO2+.
The ability of O to stabilise these high oxidation states exceeds F in this regard. Thus the highest Mn fluoride is MnF4 whereas the highest oxide is Mn2O7. The ability of oxygen to form multiple bonds to metals explains its superiority. In the covalent oil Mn2O7, each Mn is tetrahedrally surrounded by O’s including an Mn-O-Mn bridge. The tetrahedral [MO4]n- ions are known for Vv, CrvI, Mnv, MnvI and MnvII.
Comparison of the First Row Transition Metals Through The d-EIectron Configuration:
In the d° configuration of the simple ions, only Sc3+ is known to have this configuration. This configuration then occurs for those metals in which the formal oxidation states equal the total no. of 3d and 4s electron. This is true for Ti (IV), V (V), Cr (VI) and Mn (VII).
→ The d1 Configuration: Except Vanadium (IV), all others with this configuration are either reducing or undergo disproportionation. For .example, disproportionation occurs for Cr (V) and Mn (VI)
3 CrO43- + 8H+ → 2 CrO4– + Cr3+ + 4 H2O
3 MnO42- + 4H+ → 2 MnO4– + MnO3 + 2H20
→ The d2 Configuration: This configuration ranges from Ti11 which is very strongly reducing, to FeVI which is very strongly oxidizing. Vanadium (III) is also reducing.
→ The d3 configuration is shown by Chromium (III). It is quite stable and takes part in complex formation.
→ The d4 Configuration: There are really no stable species with the configuration Cr (II) is strongly reducing. ,
→ The d5 Configuration: The two important species with this configuration are Mn2+ and Fe3+, the latter may, however, be reduced to Fe2+.
→ The d6 Configuration: Iron (II) and Cobalt (III) are important species with this configuration. Iron (II) is quite stable although a mild reducing agent and cobalt (III) are stable in the presence of strong complexing reagents.
→ The d7 Configuration: The species with this configuration is cobalt (II) which is stable in aqueous solutions but gets oxidized to form CO (III) complexes in the presence of strong ligands.
→ The d8 Configuration: Nickel (II) is the most important species with this species.
→ The d9 Configuration: This configuration is found in Cu2+ compounds. It is by far the most important in the chemistry of copper.
→ The d10 Configuration: The two species Cu+ and Zn2+ are important with this configuration. Whereas Copper (I) is easily oxidized to copper (II), zinc (II) is the only state known for zinc.
General Group Trends in the Chemistry of the d-Block Metals:
Group 4: The titanium group of transition metals consists up of the elements titanium, zirconium and hafnium. The most important member of this group is titanium. It is extremely strong, has a high melting point and is resistant to corrosion. It is in great demand as a structural material. Zirconium and hafnium are silvery-white metals. The most stable oxidation state for the elements of this group is +4.
The titanium also possesses a + 3 oxidation state. The typical compounds of these elements are chlorides, TiCl4, ZrCl4, HfCl4 and oxides TiO2, ZrO2 and HfO2, ZrO2 is a refractory material. Zirconium and hafnium occur together and exhibit similar properties.
Their atomic radii (Zr = 160 pm, Hf = 159 pm) are almost equal. This is due to the reason that usually increases in size down the group is cancelled by the lanthanide contraction. Zirconium and hafnium are both important for the generation of nuclear energy.
The vanadium group (Group 5): consists of vanadium, niobium and tantalum. The most stable oxidation state for this group is + 5. Vanadium is used as an additive to steel. The most important compound of vanadium is its pentoxide, V2O5, which is used as a catalyst in many reactions. Niobium alloys are used in jet engines, Tantalum is very resistant to corrosion and is used for making apparatus in chemical plants. It is also used in surgery, as for bone pins.
The chromium group (Group 6): contains the elements, chromium, molybdenum and tungsten. The most important oxidation states for chromium are + 3 and + 6, and for molybdenum and tungsten are + 5 and + 6. The zero oxidation states for these elements arise in metal carbonyls such as Cr (CO)6.
These elements have very high melting and boiling points. Tungsten is the metal with the highest melting point. These metals are also very hard. Chromium is unreactive or passive at low temperatures because of the formation of a surface coating of oxide. It is due to this passive behaviour that chromium is used for electroplating iron to prevent rusting.
The metals of this group are very useful. Chromium is used in many ferrous alloys such as stainless steel, chrome steel, etc. It is also used for electroplating iron or some other metals to prevent corrosion. K2Cr2O7 is a very important and useful compound of chromium.
Molybdenum and tungsten form very hard alloys with steel and are used in making cutting tools. Tungsten is also used as filament in electric bulbs. Molybdenum disulphide, MoS2 acts as a lubricant because it has a layer lattice.
Group 7: the manganese group: consist of the elements manganese, technetium and rhenium. These elements exhibit all the oxidation states from 0 to + 7, the most important being + 2, + 4 and + 7 for manganese and + 4 and + 7 for technetium and rhenium.
The elements of this group have quite a high melting and boiling points.
Manganese is obtained from its oxide ores by reduction with carbon or aluminium. Manganese metal does not have any use as such but is used in the manufacture of alloys such as ferromanganese (Fe + Mn) and manganese bronze (Mn + Cu + Zn). KMnO4 is an important compound of Mn which is used as an oxidizing agent and finds so many other applications. Manganese dioxide is used as a catalyst. Rhenium is used in electronic filaments, high-temperature thermocouples and in flashbulbs.
The metals of group 8,9 and 10 are known as iron group metals: These elements are:

The first triad comprising of iron, cobalt and nickel is known as ferrous metals. These metals are ferromagnetic. Iron and cobalt exhibit oxidation states of + 3 and + 2 in their compounds while nickel compounds are generally in the + 2 oxidation state.
The elements of the second and third triad, ruthenium, rhodium, palladium, osmium, iridium and platinum are collectively known as platinum metals. These elements are relatively less abundant and exhibit a wider range of oxidation states. They are inert and serve as good catalysts.
The copper group (group 11): includes the elements copper, silver and gold. These metals are known as coinage metals. They form alloys with many metals. The most stable oxidation state for copper is + 2. For silver and gold, the oxidation state of + 1 is relatively more stable. The metals of this group have the highest electrical and thermal conductivities.
The zinc group (group 12): consists of the elements zinc, cadmium and mercury. The elements of this group show none of the characteristic properties of transition metals. The metals of this group are moderately electropositive and exhibit an oxidation state of + 2. Since their ionization energy is very high none of these metals possesses an oxidation state higher than + 2. Mercury, due to metal-metal bond also shows a formal oxidation state of +1. The elements of this group are diamagnetic. Mercury is the only metal that exists as a liquid.
Potassium Dichromate (K2Cr2O7):
Preparation: Chromite ore is fused with sodium or potassium carbonate in free access of air when the following reaction occurs.
![]()
The yellow solution of sodium chromate (Na2CrO4) is filtered and acidified with a calculated amount of sulphuric acid from which orange sodium dichromate Na2Cr2O7. 2H2O can be crystallised.
2 Na2CrO4 + 2H+ → Na2Cr2O7 + 2Na+ + H2O
Potassium dichromate is prepared by treating the solution of sodium dichromate with a calculated amount of potassium chloride.
Na2Cr2O7 + 2 KCl → K2Cr2O7 + 2 NaCl.
Orange crystals of potassium dichromate crystallise out. The dichromate and chromate are interconvertible in an aqueous solution depending upon the pH of the solution.
2 CrO42- + 2 H+ → Cr2O72- + H2O.
Cr2O72- + 2 OH– → 2 CrO42- + H2O.
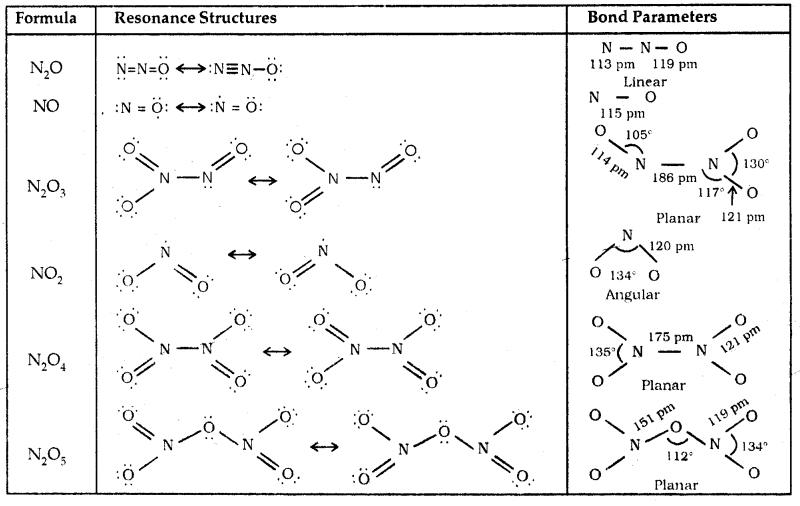
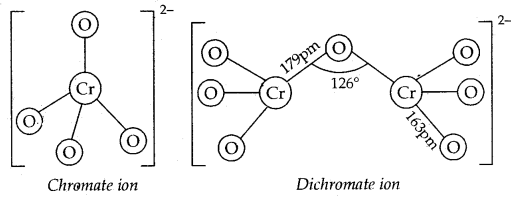
The structures of chromate ionCrO42-, and dichromate ion, Cr2O72- and shown below. Whereas CrO42- is tetrahedral sharing one comer with Cr-O-Cr bond angle of 126°.
In acidic solution, both sodium and potassium dichromates are strong oxidizing agents, the former has a greater solubility in water.
Cr2O72- + 14 H+ + 6e– → 2 Cr3+ + 7 H2O [E° = 1.33 V]
→ Acidified solution oxidizes
1. iodides to iodine
6I– → 3I2 + 6e–
2. sulphides to sulphur
3 H2S → 6 H+ + 3 S + 6e–
3. tin (II) to tin (IV)
3 Sn2+ → 3 Sn4+ + 6e–
4. iron (II) salts to iron (III)
6 Fe2+ → 6 Fe3+ + 6e–
The full ionic equation may be obtained by adding the two half-reactions. e.g.,
Cr2072- + 14 H+ + 6 Fe2+ → 2 Cr3+ + 6 Fe3+ + 7 H2O.
Potassium Permanganate, KMn04:
Preparation:
1. KMnO4 is prepared by fusion of MnO, and KOH with an oxidizing agent like KNO3.
![]()
Potassium manganate (K2MnO4) gives KMnO4 in a neutral/acidic solution.
3 MnO42- + 4H) → 2 MnO4– + MnO2 + 2H2O
2. Comfnercially, KMnO4 is prepared from MnO2 by electrolytic oxidation.
KMnO4 forms dark purple crystals. It is not very soluble in water. When heated it decomposes at 240°C.
![]()
KMnO4 shows weak temperature-dependent paramagnetism. It arises due to the coupling of the diamagnetic ground state of MnO4 ion with paramagnetic excited states under the influence of the magnetic field.
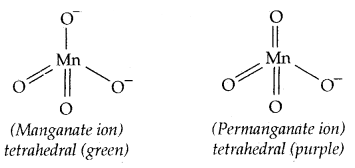
The manganate and permanganate ions are tetrahedral. The green MnO42- is paramagnetic with one unpaired electron, but MnO4– is diamagnetic.
Permanganic acid (HMnO4) can be obtained by low-temperature evaporation of its aqueous solution. It is a strong oxidizing agent and in a pure state, it is explosive above 0°C.
→ Acidified KMnO4 is a strong oxidizing agent:
MnO4– + e– → MnO42-
[Reduction half reaction]; E° = + 0.56 V
MnO4– + 4H+ + 3e– → MnO2 + 2H2O
[Reduction half reaction]; E° = + 1.69 V.
MnO4– + 8H+ + 5e– → Mn2+ + 4 H2O
[Reduction half reaction]; E° = + 1.52 V.
A few important oxidizing reactions of KMnO4 are given below.
1. In acid solutions:
(a) Iodine is liberated from potassium iodide:
10I– + 2MnO4– + 16H+ → 2Mn2+ + 8H20 + 5I2
(b) Fe2+ ion is converted to Fe3+:
5Fe2+ + MnO4– + 8H+ → Mn2+ + 4H2O + 5Fe3+
The green iron (II) solution changes to yellow iron (III) solution.
(c) Oxalate ion or oxalic acid is oxidized at 60°C:
5 C2O42- + 2MnO4–+ 16H+ → 2Mn2+ + 8H2O + 10CO2
(d) Hydrogen sulphide is oxidized, sulphur being precipitated:
H2S + 2H+ → + S2-
5S2- + 2MnO4– + 16H+ → 2Mn2+ + 8H2O + 5S
(e) Sulphurous acid or sulphite is oxidized to a sulphate or sulphuric acid:
5 SO32- + 2MnO4– + 6H+ → 2Mn2+ + 3H2O + 5SO42-
(f) Nitrite is oxidized to nitrate:
5NO2– + 2MnO4– + 6H+ → 2Mn2+ + 5NO3– + 3H2O
2. In neutral or faintly alkaline solution:
(a) A notable reaction is the oxidation of iodide to iodate:
2MnO4– + H2O + I– → 2MnO2 + 2OH– + IO3–
(b) Thiosulphate: s oxidised almost quantitatively to sulphate, a trace of thionate being formed
8MnO4– + 3S2O32- + H2O → 8MnO2 + 6SO42- + 2OH–
(c) Manganous salt is oxidised to MnOz; the presence of zinc sulphate or zinc oxide catalyses the oxidation:
2MnO4– + 3Mn2+ + 2H2O → 5MnO2 + 4H+
Note: Permanganate titrations in presence of hydrochloric acid are unsatisfactory since hydrochloric acid is oxidised to chlorine.
Uses: Besides its use in analytical chemistry, potassium permanganate is used as a favourite oxidant in preparative organic chemistry. Its uses for the bleaching of wool, cotton, silk and other textile fibres and for the decolourisation of oils are also dependent on its strong oxidising power.
→ The Inner Transition Elements (f-Block): It consists up of two series of elements.
1. Lanthanoids (58-71) which follow lanthanum (57) in the periodic table. are fourteen in number. Because La (57) closely resembles the lanthanoids, it is usually included in the study of lanthanoids and the general symbol Ln is often used.
2. Actinoids [the fourteen elements (90-103)] which follow actinium (89). A discussion of actinoids also includes actinium (Ac), besides the 14 elements.
In the case of lanthanoids, 4f orbitals/are filled up and in the case of actinoids, 5f orbitals are filled up. That is why they are called Inner Transition Elements as the last electron (also called differentiating electron) enters the antepenultimate energy level, ie.., (n – 2) f-orbitals: inner to the penultimate energy level and they form a transition series within the transition series (d-block elements)
Lanthanides or Landhanoids or Lanthanoness: In these elements, the last electron enters the 4f-orbitals and is also referred to as the first inner transition series. Earlier they were called rare earth.
Actinides or Actinoids or Actions: In these elements, the last electron enters one of the 5f-orbitals and is also required as the second inner transition series.
The study of lanthanoids is easier because they show only one stable oxidation state (+ 3). On the other hand, the chemistry of actinoids is much more complicated partly because they show a wide range of oxidation states and partly because they are radioactive.
→ Electronic Configuration: General E.C. of lanthanoids and actinoids is (n – 2) f1-14 (n – 1) d0-1 ns2. Thus they have three incomplete shells, viz., (n – 2), (n – 1) and nth. Their electronic config. is given below.
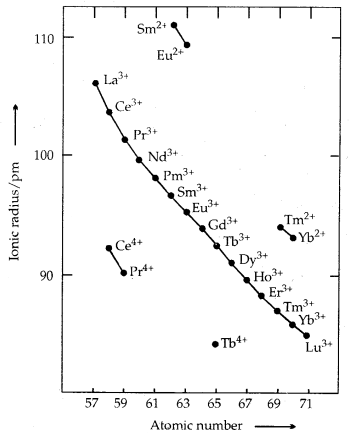
Trends in Ionic Radii of Trivalent Lanthanoids
Table: Electronic configurations and radii of lanthanum and lanthanoids:
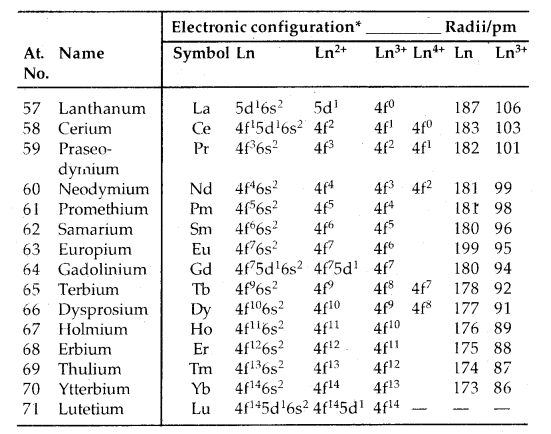
Only electrons outside [Xe] core are indicated.
The overall decrease in atomic and ionic radii from lanthanum to lutetium (the lanthanoid contraction) is a unique feature in the tire chemistry of lanthanoids. It is due to the imperfect shielding of one 4f electron from another 4f electron due to the highly diffused shape of f-orbitals. With the increase in nuclear charge, there is a fairly regular decrease in their sizes.
The cumulative effect of the contraction of the lanthanoid series called lanthanoid contraction causes the radii of the third transition series to be very similar to those of the correspond ing members of the 2nd series. As a.result Zr (160 pm) and Hf (159 pm) have identical radii and so exist together.
Thus Zr and Hf face difficulty in their separation.
Colour and Paramagnetism: Many trivalent lanthanoids are coloured both in the solid-state and in aqueous solutions. It is due to the presence of f-electrons. Neither Lu3+ ion (f°) nor Lu3+ (f14) shows any colour. All others (having f1 to f13 arrangement) show colour. The lanthanoid ions other than f° (La3+, Ce4+) type and the f14 type (Yb2+, Lu3+) are all paramagnetic due to the presence of unpaired electrons. Paramagnetism is maximum in neodymium.
→ Ionisation Enthalpies: The IE1 and IE2 are around 600 kJ mol-1 and 1200 kJ moH comparable with those of calcium. The values of IE3 indicate that the exchange energy considerations impart a degree of stability to empty, half-filled and completely filled f-level (f°, f7, f14). It is evident from the abnormally low values of IE3 of lanthanum, gadolinium and lutetium.
→ Oxidation States: All the lanthanoids show a + 3 oxidation state which is most significant: Some of them show + 2 and + 4 oxidation states also as ions in solution or in solid compounds. This irregularity arises mainly from the extra stability of empty, half-filled or completely filled f-subshell. CeIV [16Rn-noble gas] is formed easily, but it is a strong oxidant reverting to the common oxidation state of + 3.
Ce4+ + e– → Ce3+] Reduction half reaction
E° for the above is = + 1.74 V
If can oxidise water, but the reaction rate is slow. Pr, Nd, Tb and Dy also exhibit + 4 states in oxides MOr Eu2+ is formed by losing the two s-electrons (F). However, Eu2+ is a strong reducing agent changing to the common oxidation state + 3.
EU2+ → EU3+ + e–
Similarly Yb2+ [f14] is a reductant. Tb (IV) [f7] is an oxidant.
Tb4+ + e- → Tb3+
Samarium also shows oxidation states of + 2 and + 3 like Eu.
Properties and Uses:
1. All the lanthanides are silvery-white soft metals and tarnish rapidly in the air. Hardness increases with increasing atomic number, Samarium is steel hard.
2. M.Pts are in the range of 1000 to 1200 K, but Sm melts at 1623 K
3. They have a metallic structure.
4. They are good conductor of heat and electricity.
5. In their chemical behaviour, lanthanoids are generally similar to calcium (Ca = 20), but with increasing atomic number, they behave more like aluminium.
6. Values for E° for the reduction half-reaction.
Ln3+ (aq) + 3e– → Ln (s) are in the range of – 2.2 to – 2.4 V except for Ln = Eu, for which it is – 2.0 V,
7. The metals combine with hydrogen when heated gently.
2 Ln + 3 H2 → 2 Ln H3
8. When heated in carbon, carbides of the type Ln3C, Ln2C3 and LnC2 are formed.
9. They liberate H2 gas from dilute acids.
2 Ln + 6HCl (dil.) → 2 LnCl3 + 3H2I
10. They bum in halogens to form halides.
2 Ln + 3X2 → 2 LnX3
11. They form oxides and hydroxides of the type M2O3 and M (OH)3. They are basically like alkaline earth metal oxides and hydroxides. A summary of the chemical reactions of lanthanoids are given below:
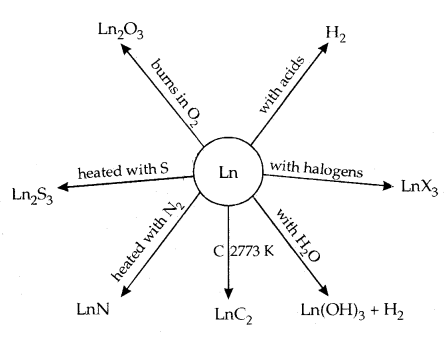
The best use of the lanthanoids is for the production of alloy steels for plates and pipes. A well-known alloy is Misch Metall which consists of a lanthanoid metal (~ 95%) and iron (~ 5%) and traces of S, C, Ca and Al. It is used to produce bullets, shell and lighter flint. Mixed oxides are used as catalysts in cracking. Some Ln oxides are used as phosphors in TV screens.
→ The Actinoids: The elements which follow actinium (89) in the periodic table are called actinoids [Starting from Th – 90 to Lr – 103.]
→ Actinoids are radioactive elements. Earlier members have relatively long half-lives and later ones from day to 3 minutes for Lr. This makes their study difficult.
→ Electronic Configuration: All the actinoids have a 7s2 electron configuration and variable occupancy of the 5f and 6d subshells. The irregularities in the electronic configuration of actinoids like those in lanthanoids are related to the stabilities of 5f°, 5f7 and 5f14. 5f orbitals participate in bonding to a greater extent.
→ Oxidation states: There is a greater range of oxidation states shown by actinoids due to the comparable energies of 5f, 6d and 7s levels.
The electronic configurations and oxidation states of actinoids are in the following tables.
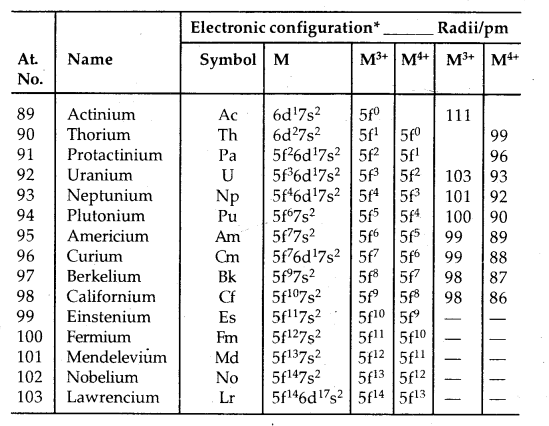
Table: Oxidation states of actinium and actinoids:
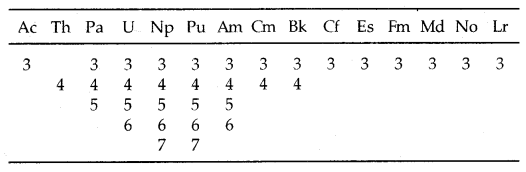
Unlike 4f orbitals of lanthanoids which are buried, 5f orbitals of actinoids participate in bonding to a far greater extent. In addition to showing an oxidation state of + 3 like lanthanoids, actinoids also show oxidation states of + 4, + 5, + 6 and + 7. Actinoids which show several oxidation states [Np, Pu, Am] make it difficult to review their chemistry.
Physical and Chemical reactivity of actinoids
- Actinoids are all silvery-white metals.
- They are highly reactive metals, especially when finely divided.
- Boiling water reacts with them to give a mixture of oxides, hydrides.
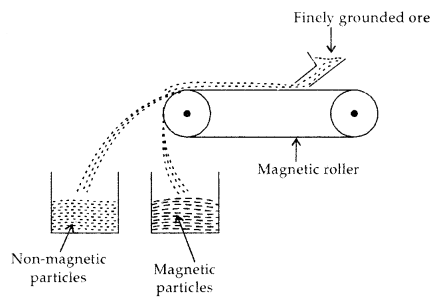
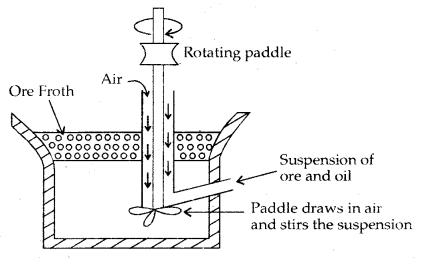
Some applications of d-Block elements
- Iron and Steel are the most important construction materials.
- Compounds like TiO are prepared for use in the pigment industry.
- Mn02 is used in dry battery cells. The battery industry also requires Zn and Ni/Cd.
- Cu, Ag and Au are coinage metals.
- Many of the metals and their compounds find use as catalysts like finely divided Ni, V2O5, TiCl4, Fe etc.
- PdCl2 is used in the Wacker process for the oxidation of ethyne to ethanol
- Ni-complexes are used in the polymerisation of alkynes and other organic compounds like benzene.
- AgBr is used in the photographic industry.