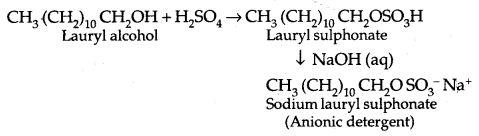
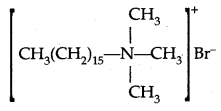
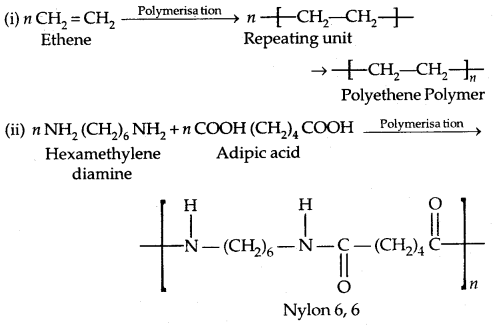
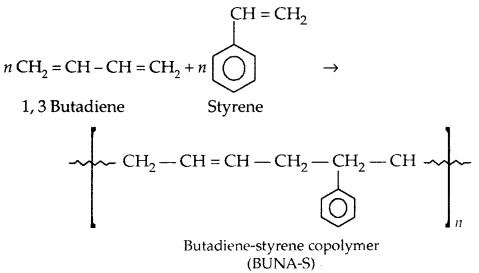
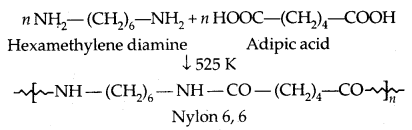
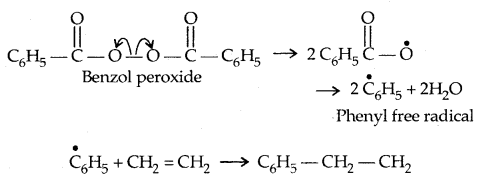
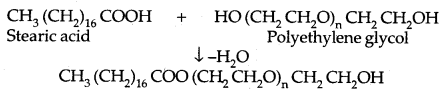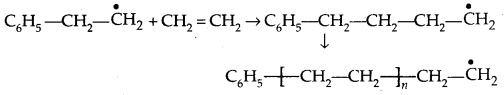By going through these CBSE Class 11 Physics Notes Chapter 2 Units and Measurement, students can recall all the concepts quickly.
Units and Measurement Notes Class 11 Physics Chapter 2
→ Physical Quantity = numerical value × unit = nu
→ Numerical value (n) ∝ \(\frac{1}{\text { size of unit(u) }}\)
→ Physical quantities which are independent of each other are called fundamental quantities.
→ Units of fundamental quantities are called fundamental units.
→ There are four systems of units namely FPS, CGS, MKS, and S.I. system.
→ 1 a. m.u.= 1.66 × 10-27kg.
→ The product of n and u is called the magnitude of the physical quantity.
→ Force, thrust, and weight have the same SI unit, i.e. Newton.
→ Pressure, stress, and coefficient of elasticity have the same SI unit, i.e. Pascal.
→ The standard unit must not change with time and space. That is why the atomic standards for length and time have been defined.
→ The dimensions of many physical quantities especially those of heat, electricity, thermodynamics, and magnetism in terms of mass, length, and time alone become irrational, so SI is adopted which uses 7 basic units and two supplementary units.
→ The first conference on weights and measures was held in 1889.
→ Sevres near Paris is the headquarter of the International Bureau of Weights and Measures.
→ SI system was first adopted in the 11th general Conference of Weights and Measures in 1960.
→ S.I. system is also known as the rationalized M.K.S. system.
→ The various units of the S.I. system are rational in nature.
→ The various units of the S.I. system are coherent in nature.
→ It is wrong to say that the dimensions of force are [MLT-2]. On the other hand, we should say that the dimensional formula for force is [MLT-2].
→ The dimensional formula for the dimensionless physical quantity is written as [M°L°T°].
→ The dimensions of a physical quantity don’t depend on the system of units.
→ The dimensional formula is very helpful in writing the unit of a physical quantity in terms of the basic units.
→ The pure numbers are dimensionless.
→ Physical quantities defined as the ratio of two similar quantities are dimensionless.
→ The physical relations involving logarithm, exponential, trigonometric ratios, numerical factors, etc. cannot be derived by the method of dimensional analysis.
→ Physical relations involving addition or subtraction sign cannot be derived by the method of dimensional analysis.
→ If units or dimensions of two physical quantities are the same, these need not represent the same physical characteristics.
→ Torque and work have the same dimensions but have different physical characteristics.
→ Measurement is most accurate if its observed value is very close to the true value.
→ Significant figures are the number of digits up to which we are sure about their accuracy.
→ Significant figures don’t change if we measure a physical quantity in different units.
→ Significant figures retained after the mathematical operation (like addition, subtraction, multiplication, or division) should be equal to the minimum significant figures involved in any physical quantity in the given operation.
→ Error = Actual value: Observed value.
→ Absolute error: Δxi = \(\overline{\mathrm{x}}\) – xi
→ The absolute error in each measurement is equal to the least count of the measuring instrument.
→ Mean absolute error
Δx = \(\frac{1}{x} \sum_{i=1}^{n}\)(Δx1)
→ When we add or subtract two measured quantities, the absolute error in the final result is equal to the sum of the absolute errors in the measured quantities.
→ When multiply or divide two measured quantities, the relative error in the final result is equal to the sum of the relative errors in the measured quantities.
→ For greater accuracy, the quantity with higher power should have the least error.
→ Smaller is the least count higher is the accuracy of measurement.
→ The relative error is a dimensionless quantity.
→ The unit and dimensions of the error are the same as that of the quantity itself.
→ The larger the number of significant digits after the decimal point in measurement, the higher is the accuracy of measurement.
→ Physical quantities: Physical quantities may be defined as the quantities in terms of which physical laws can be expressed and which can be measured directly or indirectly.
→ Subjective methods: The methods of measurement which depend on our senses are called subjective methods.
→ Objective methods: The methods of measurement which make use of scientific instruments are called objective methods.
→ Fundamental quantities: The quantities which are independent of each other and which are not generally defined in terms of other physical quantities are known as fundamental or basic quantities.
→ Derived quantities: The quantities whose defining operations are based on the fundamental physical quantities are called derived quantities.
→ Unit: A unit is defined as the reference standard of measurement.
→ If a number is without a decimal point and ends in one or more zeros, then all the zeros at the end of the number may not be significant.
→ To make the number, of Significant digits clear, it is suggested that the number may be written in exponential form.
→ For example, 20300 may be expressed as 203.00 × 102, to suggest that all the zeros at the end of 20300 are significant.
→ Fundamental or basic units: The basic units are those which can neither be derived from one another nor can be resolved into further units! For example units of length, mass and time, etc. These are 7 in number.
→ Derived units: The units of all those physical quantities which can be expressed in terms of fundamental units are called derived units. For example, units of velocity, force, and energy, etc.
→ Size of a physical quantity: The size of a physical quantity is determined by a unit and the number of times that unit is to be repeated to represent the complete quantity.
Size of a physical quantity = nu;
n = number of times the chosen unit is contained in the physical quantity,
u = size of the unit.
→ System of units: Complete set of units both for fundamental and derived quantities is known as a system of units.
→ S.I. Units: Systeme international of units, in short, is called S.I. units.
It has seven fundamental units namely
- unit of length is meter (m),
- kilogram (kg) unit of mass,
- second (s) unit of time,
- ampere (A) unit of current,
- Kelvin (K) unit of temperature,
- Candela (cd) unit of light intensity and
- mol (mole) for a unit of amount of substance.
→ There are two supplementary units for measuring: (a) plane angle and solid angle. These are radian (rad) and steradian (sr) respectively.
→ θ(rad) = \(\frac{\text { arc }}{\text { radius }}=\frac{l}{r}\)
→ Ω(sr) = \(\frac{\text { surface area }}{(\text { radius })^{2}}=\frac{\Delta \mathrm{A}}{\mathrm{r}^{2}}\)
→ Length: It is defined as a measure of separation between two points in space.
→ Mass: It is the amount of substance contained in the body. Inertial mass: It is the mass of the body which is a measure of inertia F
∴ m = \(\frac{F}{a}\)
→ Gravitational mass: It is the mass of the body that determines the gravitational pull due to the earth acting on the body.
∴ m = \(\frac{W}{g}\)
→ Fermi (F): It is a unit of extremely small distances:
1 F = 10-15 m.
→ Angstrom (A): It is the unit of length at the atomic level:
1 A = 10-10 m ,
→ Astronomical unit (AU): It is the unit of length at a large scale:
1 A.U. = 1.496 × 1011 m= 1.5 × 1011 m.
→ Light year- It is defined as the distance traveled by light in one year
1 L.Y. = 9.46 × 1015 m.
→ Meter (m): Metre is the unit of length and is defined as the space occupied by 1,650,763.73 wavelengths of orange-red light emitted by krypton: 86 kept “at the triple point of nitrogen (radiation emitted due to transition between the levels 2P10 and 5d5).
→ Kilogram (kg): Kilogram is the unit of measurement of mass. It is the mass of international prototype platinum-iridium cylinders kept in the International Bureau of Weights and Measures at Sevres, France.
→ Second(s): It is the unit of time. A second is the duration of time corresponding to 9,192,631,770 vibrations corresponding to the transition between two hyperfine levels of cesium-133 atom in the ground state.
→ Ampere(A): An ampere of current is defined as the constant current, which when flowing through two straight parallel conductors of infinite length and negligible area of cross-section placed lm apart in air produces a force of 2 × 10-7 Nm-1.
→ Parsec: This unit is used to measure very large distances i.e., the distance between stars or galaxies.
1 Parsec = 3.08 × 1016m
→ Atomic mass unit (AMU): It is the unit of mass at the atomic and subatomic levels.
1 amu = \(\frac{\left(\text { mass of }_{6} C^{12} \text { atom }\right)}{12}\)
→ Dimensions: The dimensions of a physical quantity are the powers to which the fundamental units of length, mass and time have to be raised to obtain its units, e.g., dimensions of force [MLT-2] are 1 in mass 1 in length and -2 in time.
→ Dimensional formula: Dimensional formula of a physical quantity is defined as the expression that indicates which of the fundamental units of mass, length, and time appear into the derived unit of that physical quantity and with what powers.
→ Dimensional equation: The equation obtained by equating the physical quantity to its dimensional formula is called the dimensional equation of that physical quantity.
→ Dimensional variables: The variable quantities which have dimensions are called dimensional variables! For example, velocity, force, momentum, etc.
→ Dimensionless variables: These are variable physical quantities that do not have dimensions. For example, relative density, specific heat, strain, etc.
→ Dimensional constants: Those constants which have dimensions are called dimensional constants. For example, gravitational constant, Planck’s constant.
→ Dimensionless constants: Those constants which do not have, dimensions are dimensionless constants. For example, all trigonometric functions, natural numbers 1, 2, 3…. π, e.
→ Significant figure: The significant figures are a measure of the accuracy of a particular measurement of a physical quantity. Significant figures in measurement are those digits in a physical quantity that are known reliably plus the one-digit which is uncertain.
→ Error: It is the difference between a true and measured value of a physical quantity.
→ Discrepancy: The difference between the two measured values of a physical quantity is known as a discrepancy.
→ Constant error: It is an error in measurements. It arises due to some constant causes such as faulty calibration on the instrument. This error remains constant in all observations.
→ Systematic error: This error is also a measurement error. The error is one that always produces an error of the same sign. This error may be due to imperfect technique, due to alteration of the quantity being measured, or due to carelessness and mistakes on the part of the observer.
→ Instrumental error: This is a constant type of error. These are errors of an apparatus and that of the measuring instruments used e.g., zero error in vernier calipers or screw gauge.
→ Error due to least count: This also is another type of constant error. The error due to the limitations imposed by the least counts of the measuring instruments comes under this heading.
→ Observational or Personal Error: This is a subheading of systematic error. This error is due to the experimental arrangement or due to the habits of the observer.
→ Error due to physical conditions: These errors are due to the experimental arrangement or due to the habits of the observer. These are also systematic errors.
→ Error due to unavoidable situations: These errors are due to the imperfectness of the apparatus or of non-availability of ideal conditions.
→ Random errors: The errors due to unknown causes are random errors.
→ Gross error: These types of errors are because of the carelessness of the observer.
These errors may be due to
- negligence towards sources of error due to overlooking of sources of error by the observer;
- the observer, without caring for least count, takes wrong observations;
- wrong recording of the observation.
→ Absolute error: The magnitude of the difference between the true value and the measured value is called absolute error.
→ A relative error: It is defined as the ratio of the mean absolute error to the true value.
→ Percentage error: The relative error expressed in percentage is percentage error.
→ Standard error: The error which takes into account all the factors affecting the accuracy of the result is known as the standard error.
→ Standard deviation: The root means the square value of deviations (the deviation of different sets of observations from the arithmetic mean) is known as standard deviation.
Standard deviation σ = \(\sqrt{\frac{\left(\mathrm{x}_{1}-\overline{\mathrm{x}}\right)^{2}+\left(\mathrm{x}_{2}-\overline{\mathrm{x}}\right)^{2}+\left(\mathrm{x}_{\mathrm{n}}-\overline{\mathrm{x}}\right)^{2}}{\mathrm{n}}}=\sqrt{\frac{\mathrm{S}}{\mathrm{n}}}\)
→ Probable error: The error calculated by using the principle of probability are probable errors. According to Bessels formula
→ Probable error e = ± 0.6745\(\sqrt{\frac{S}{n(n-1)}}\)
→ Standard error = \(\sqrt{\frac{\mathrm{S}}{n(n-1)}}\)
Important Formulae:
→ t = Size of oleic acid molecule = thickness of film of oleic acid
= \(\frac{\text { Volume of film }}{\text { Area of film }}\)
→ Inertial mass determination:
\(\frac{m_{1 i}}{m_{2 i}}=\frac{T_{1}^{2}}{T_{2}^{2}}\) where T1 and T2 are of the time of oscillation of inertia balance with inertial masses.
→ Gravitational mass determination:
\(\frac{\mathrm{w}_{1}}{\mathrm{w}_{2}}=\frac{\mathrm{m}_{\mathrm{g}_{1}}}{\mathrm{~m}_{\mathrm{g}_{2}}}\)
where mg1 and mg2 are gravitational masses.
→ Height by triangulation method:
- The height of an accessible object, h = x tanθ, where θ = angle of elevation of the object at the point of observation at a distance x from it.
- The height of the inaccessible object is:
h = \(\frac{x}{\cot \theta_{2}-\cot \theta_{1}}\)
where θ1 and θ2 are the angles made at two points of observation at distance x from each other.
→ Distance of stars (parallax method):
S = \(\frac{\mathrm{b}}{\theta}\), θ = Φ1, + Φ2, where Φ1, and Φ2, are the angles subtended by star on observer on Earth with an interval of 6 months.
θ = angle of parallax.
b = basis = distance between two points on the surface of earth.
→ n2 = n1 \(\left[\frac{\mathrm{m}_{1}}{\mathrm{~m}_{2}}\right]^{a}\left[\frac{\mathrm{L}_{1}}{\mathrm{~L}_{2}}\right]^{b}\left[\frac{\mathrm{T}_{1}}{\mathrm{~T}_{2}}\right]^{\mathrm{c}}\)
→ Distance by reflection method (Radar) is given by
d = \(\frac{c \times t}{2}\) where
c = velocity of light in vacuum
t = time in which it is covered twice.
→ d = \(\frac{\mathrm{ut}}{2}\) for Sonar, where u = velocity of sound waves.
→ Diameter of moon is D = Sθ, where θ is the angle made by the diameter of moon at the observer, S = distance of observer from the moon, D = diameter of moon or an astronomical object.
→ Radius of atom is r = \(\left(\frac{M}{2 \pi N \rho}\right)^{1 / 3}\)
Where N = Avogadro’s number
M = molecular weight of the substance
ρ = density of substance.
→ Relative error = \(\frac{\Delta \mathrm{x}}{\mathrm{x}}\)
→ % error = \(\frac{\Delta \mathrm{x}}{\mathrm{x}}\) × 100
→ Error in sum or difference form, ± Δz = ± Δp ± Δq
→ Maximum error in product or quotient form, \(\frac{\Delta z}{z}=\frac{\Delta p}{p}+\frac{\Delta q}{q}\)
→ % Error in power form,\(\frac{\Delta \mathrm{z}}{\mathrm{z}}\) × 100 = n\(\frac{\Delta \mathrm{p}}{\mathrm{p}}\) × 100