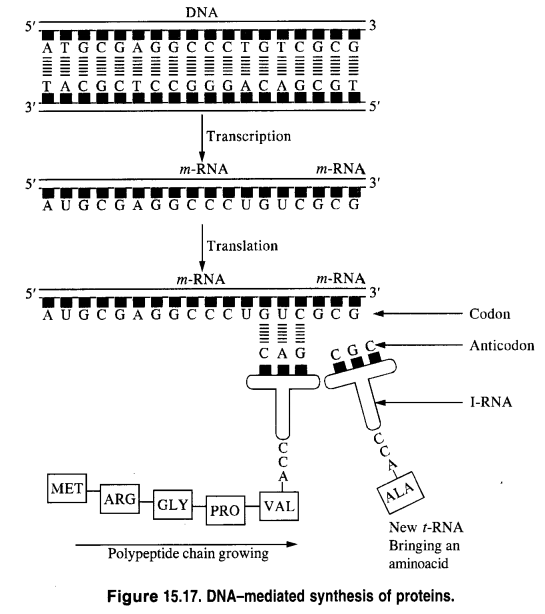
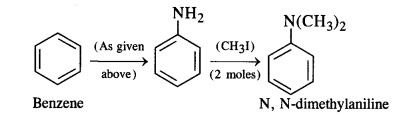
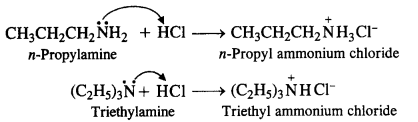
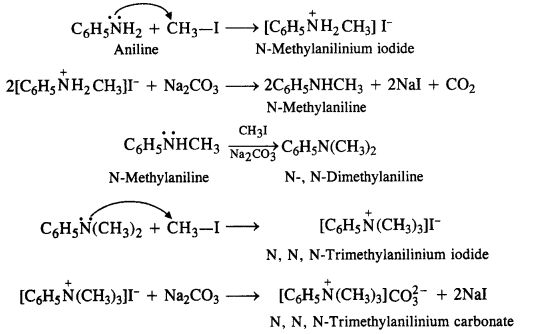
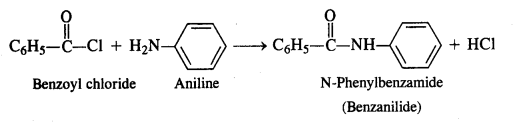
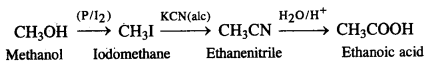
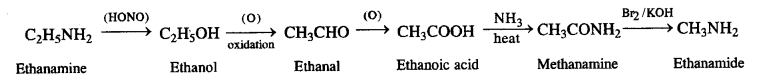
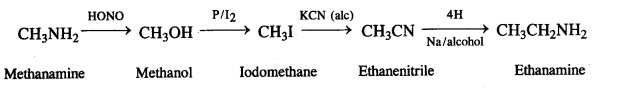
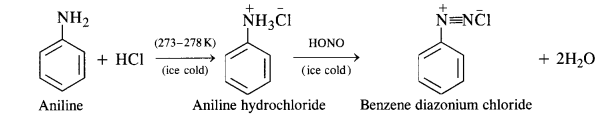
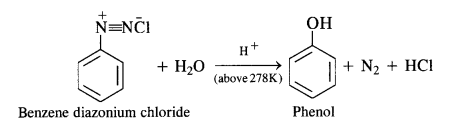
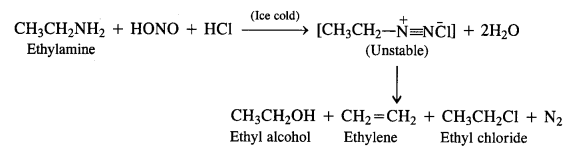
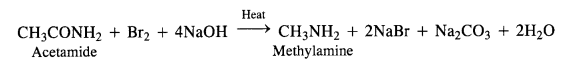
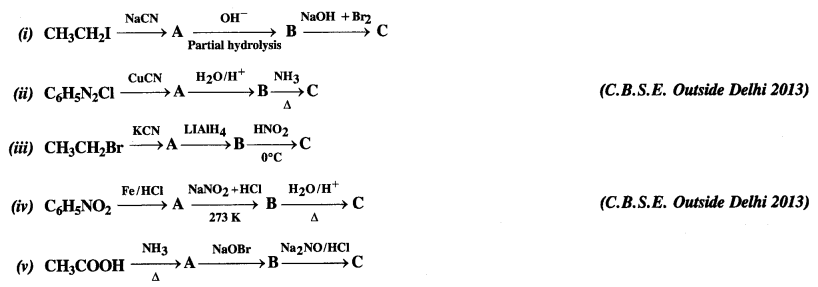
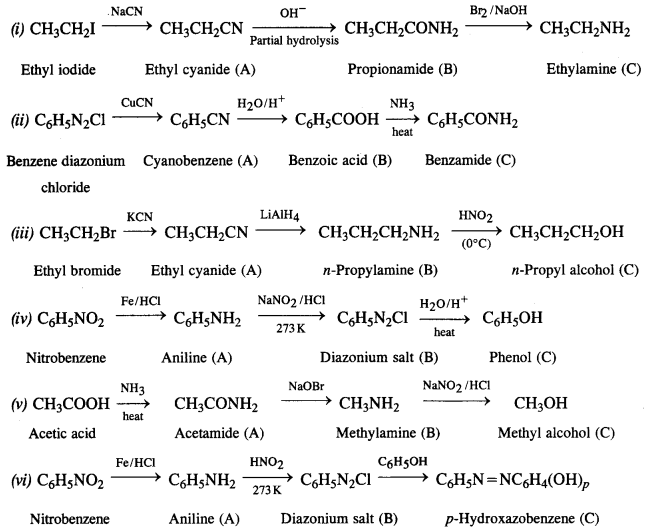
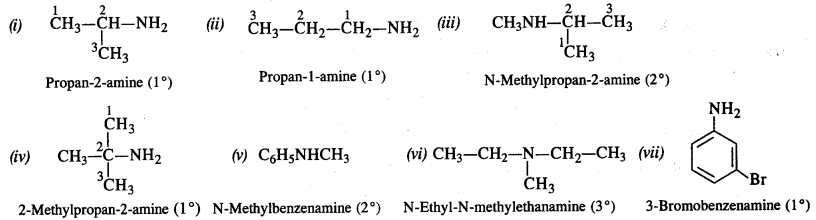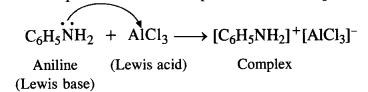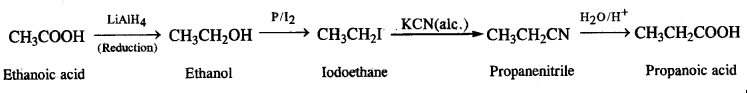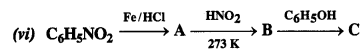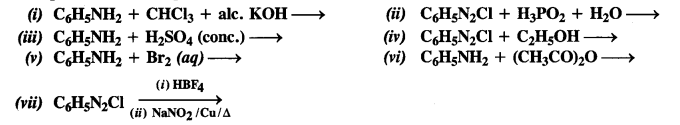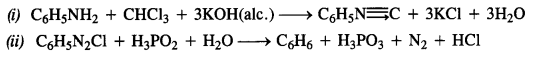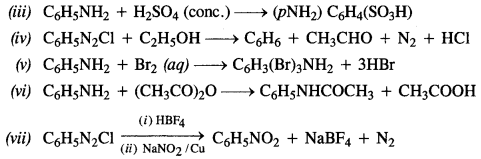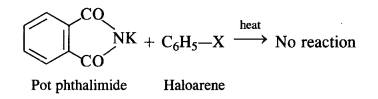NCERT Solutions for Class 12 History Chapter 7 An Imperial Capital: Vijayanagara are part of NCERT Solutions for Class 12 History. Here we have given NCERT Solutions for Class 12 History Chapter 7 An Imperial Capital: Vijayanagara.
| Board | CBSE |
| Textbook | NCERT |
| Class | Class 12 |
| Subject | History |
| Chapter | Chapter 7 |
| Chapter Name | An Imperial Capital: Vijayanagara |
| Number of Questions Solved | 9 |
| Category | NCERT Solutions |
NCERT Solutions for Class 12 History Chapter 7 An Imperial Capital: Vijayanagara
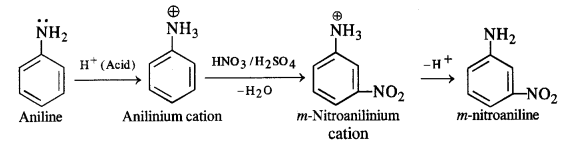
Question 1.
What have been the methods used to study the ruins of Hampi over the last two centuries ? In what way do you think they would have complemented the information provided by the priests of the Virupaksha temple ?
Solution :
(a) The methods used to study the ruins of Hampi over the last two centuries were as given below:
- Colonel Colin Mackenzie, an engineer, antiquarian and an employee of the English East India Company discovered the ruins at Hampi in 1800. He prepared the first survey map of site.
- From 1856, photographers began to record the monuments which enabled scholars to study them.
- Since 1836, epigraphists began collecting several dozen inscriptions found at the temples at Hampi.
- Thereafter the historians collated the information from above sources with accounts of foreign travellers and other literature written in Telugu, Kannada, Tamil and Sanskrit.
(b) The above methods would have complemented the information provided by the priests of the Virupaksha temple because that was based on the memories of the priests. The earlier information was corroborated by the inscriptions, photographs, maps and accounts of foreign travellers and other material.
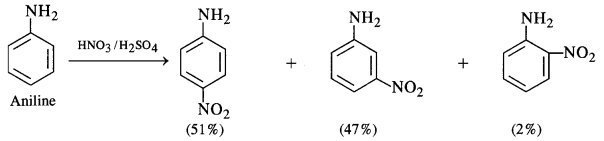
Question 2.
How were the water requirements of Vijayanagara met ?
Solution :
The water requirements of Vijayanagara were met in the following ways :
- Its location is the natural basin formed by the river Tungabhadra which flows in a north-easterly direction. The stunning granite hills form a girdle around the city. A number of streams flow down to the river from these rocky outcrops.
- The embankments were built along these streams to create reservoirs of varying
sizes. - Tanks were built to store rainwater and conduct it to the city. The most important tank built is now called Kamalapuram tank. Water from this tank irrigated fields nearby as well as was also conducted through a channel to the “royal centre”.
- The Hiriya canal drew water from a dam across the Tungabhadra and irrigated the cultivated valley that separated the “sacred centre” from the “urban core”.
Question 3.
What do you think were the advantages and disadvantages of enclosing agricultural land within the fortified area of the city?
Solution :
Advantages of enclosing agriculture land within fortified area:
(i) It had an elaborate canal system which drew water from the Tungabhadra to provide irrigation facilities.
(ii) It enclosed agricultural tracts, cultivated fields, gardens, and forests.
(iii) This enclosure saved crops from being eaten by wild animals.
(iv) In the medieval period, sieges were laid to starve the defending armies into submission. These sieges lasted for many months or many years. So the rulers of Vijayanagara adopted and elaborated a strategy to protect the agricultural belt and built large granaries.
Disadvantages
(i) This system was very expensive.
(ii) During adverse, circumstances this system proved inconvenient to the farmers.
(iii) The farmers had to seek the permission of gate-keeper to reach their field.
(iv) If enemy encircled the field the farmer could not look after their field.
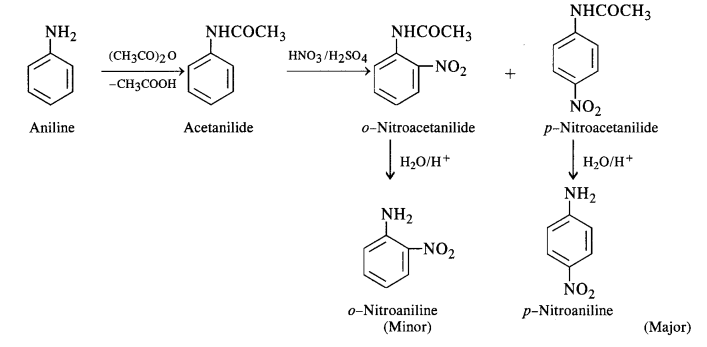
Question 4.
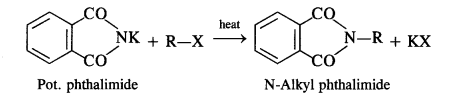
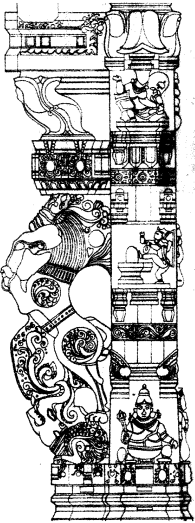
Figure given below is an illustration of another pillar from the Virupaksha temple. Do you notice any floral motifs? What are the animals shown? Why do you think they are depicted? Describe the human figures shown.
Solution :
(a) There are many floral motifs.
(b) Horses and elephants have been shown in the pillar.
(c) The Vijayanagara kings competed with contemporary rulers including the Sultans of the Deccan and the Gajapati rulers of Orissa – for control of the fertile river valleys and resources generated by lucrative overseas trade. The kingdom remained in constant state of military preparedness. So, the kings paid attention to improve harbours and encouraged its commerce so that horses, elephants, precious gems, etc. are freely imported. Thus, due to the importance of horses and elephants in the warfare, these animals had been depicted on the pillars.
(d) The images of gods have been shown in the pillar. One devotee has also been shown before a Shiva linga.
Question 5.
What do you think was the significance of the rituals associated with the mahanavami dibba?
Solution :
The mahanavami dibba was one of the most impressive platforms in the “king’s palace”. It was located on one of the highest points in the city. Rituals associated with the structure probably coincided with mahanavami (literally the great ninth day) of the ten day Hindu festival during the autumn months of September and October, known variously as Dusehra (northern India), Durga Puja (in Bengal) and Navaratri or Mahanavami (in peninsular India). The Vijayanagara kings displayed their prestige, power and suzerainty on this occasion.
The ceremonies such as worship of the image, worship of the state horse, the sacrifice of buffaloes and other animals were performed on this occasion. Dances, wrestling matches, processions of caparisoned horses, elephants, chariots, soldiers and ritual presentations were held before the kings, guests, the chief nayakas were held. On the last day, the king inspected his army and the armies of the nayakas who brought rich gifts for the king as well as the stipulated tribute. Thus, there was great significance of the rituals associated with the mahanavami dibba.
Question 6.
Discuss whether the term “royal centre” is an appropriate description for the part of the city for which it is used.
Solution :
The term “royal centre” is an appropriate description for the part of the city for which it is used because the Royal center had more than 60 temples. Most of these temples were constructed by the ruler of Vijayanagara Empire to express their supremacy. The royal centre had 30 palaces. These were made of perishable material. A brief description of the building of Royal centre are as given below:
(i) One of the most beautiful buildings in the royal centre is the Lotus Mahal. It was named by British travellers in the nineteenth century. While the name is certainly romantic, historians are not quite sure what the building was used for. One suggestion, found in a map drawn by Mackenzie, is that it may have been a council chamber, a place where the king met his advisers.
(ii) Most temples were located in the sacred centre. One of the most spectacular of these is the Hazara Rama Temple. This was probably meant to be used only by the king and his family.
Question 7.
What does the architecture of buildings like the Lotus Mahal and elephant stables tell us about the rulers who commissioned them ?
Solution :
The Lotus Mahal had nine towers – a high central one, and eight along the sides. Although it is not clear for what the building was used for but according to Mackenzie, it may have been a council chamber, place where the king met his advisers. Elephant stables were located close to the Lotus Mahal.
The architecture of Lotus Mahal tells us that the rulers used to consult their advisers on various issues and problems and meetings were held in the council chamber i.e., Lotus Mahal. The construction of “elephant stables” shows that the rulers took interest in the trade of elephants as well as in keeping them properly because elephants were very important factor in the warfare. It is perhaps one of reasons that elephants and horses have been depicted on the panels of the Hazara Rama temple.
Question 8.
What are the architectural traditions that inspired the architects of Vijayanagara ? How did they transform these traditions ?
Solution :
(a) The architectural traditions that inspired the architects of Vijayanagara were as given below :
- Prior to Vijayanagara, Cholas in Tamil Nadu and the Hoysalas in Karnataka had extended patronage to elaborate temples such as the Brihadishvara temple, Thanjavur and the
Chennakeshava temple at Belur. The rulers of Vijayanagara built on these traditions and carried them literally to new heights. - Like Indo-Islamic architecture, there was an arch on the gateway leading into fortified settlement as well as dome over the gate.
- The architecture of tombs and mosques located in the urban core resembles that of the mandapas found in the temples of Hampi.
- The Pallavas, Chalukyas, Hoysalas and Cholas encouraged temple building as a means of associating themselves with the divine – the deity was identified with the king. The choice of the site Vijayanagara was perhaps inspired by the existence of the shrines of Virupaksha and Pampadevi.
- The arches in the Lotus Mahal were inspired by Indo-Islamic technique.
(b) They transformed these traditions in the followings ways :
- In the fortifications, according to Abdur Razzaq, no mortar or cementing agent was employed. The stone blocks were wedge shaped, which held them in place, and the inner portion of the walls was of earth packed with rubble.
- Royal portrait sculpture was innovated and developed. It was displayed in temples and the king’s visits to temples were treated as important state occasions.
- In temple architecture, new features were structures of immense scale best exemplified by the Raya gopurams or royal gateways. They often dwarfed the towers on the central shrines and signalled the presence of the temple from a great distance. Other features include mandapas or pavilions and long, pillared corridors.
Question 9.
What impressions of the lives of the ordinary people of Vij ay an agar a can you cull from the various descriptions in the chapter ?
Solution :
The various descriptions in this chapter give the following impression of the lives of the ordinary people of Vijayanagara :
- Horses were imported from Arabia and Central Asia. This trade was done by the traders and local communities of merchants i.e., kudirai chettis or horse merchants.
- There were markets dealing in spices, textiles and precious stones.
- Vijayanagara boasted of a wealthy population that demanded high-value exotic goods.
- Portuguese traveller Barbosa described the houses of ordinary people, which have not survived as “the other houses of the people are thatched, but nonetheless well-built and arranged according to occupations, in long streets with many open places”. Besides this there is little archaeological evidence of the houses of ordinary people.
- There were numerous shrines and small temples which implies that there were variety of cults, supported by different communities.
- There were wells, rainwater tanks, temple tanks which may have served as sources of water to the ordinary town dwellers.
- Paes gives us a vivid description of a bazaar. He states that the provisions, such as rice, wheat, barley, etc. were available cheaply and abundantly. This means that the life of the ordinary people was good and they did not suffer for want of essential things.
We hope the NCERT Solutions for Class 12 History Chapter 7 An Imperial Capital: Vijayanagara help you. If you have any query regarding NCERT Solutions for Class 12 History Chapter 7 An Imperial Capital: Vijayanagara, drop a comment below and we will get back to you at the earliest.