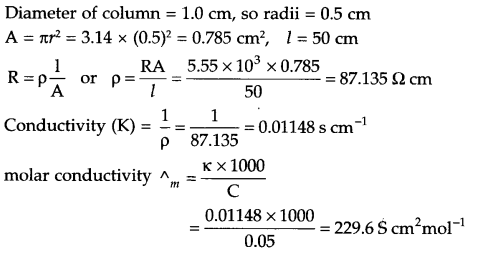
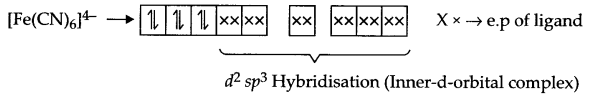
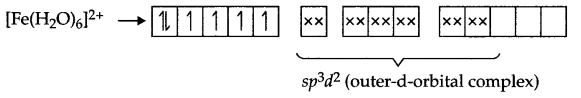
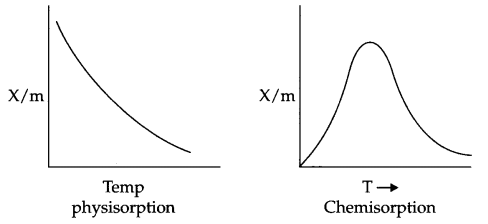
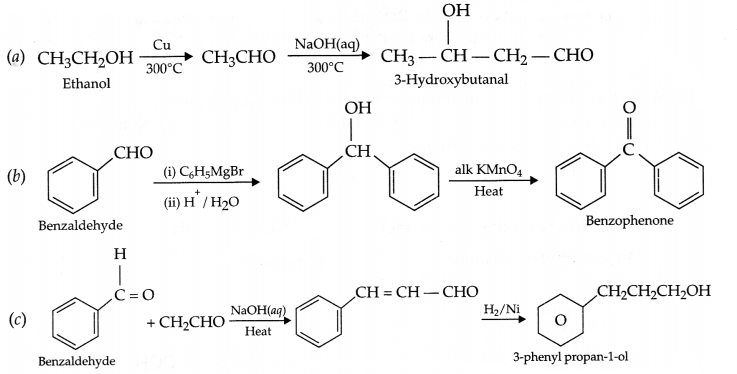
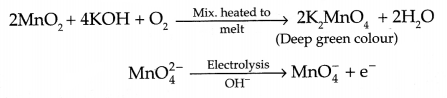
CBSE Sample Papers for Class 12 Political Science Paper 4 are part of CBSE Sample Papers for Class 12 Political Science. Here we have given CBSE Sample Papers for Class 12 Political Science Paper 4.
CBSE Sample Papers for Class 12 Political Science Paper 4
| Board | CBSE |
| Class | XII |
| Subject | Political Science |
| Sample Paper Set | Paper 4 |
| Category | CBSE Sample Papers |
Students who are going to appear for CBSE Class 12 Examinations are advised to practice the CBSE sample papers given here which is designed as per the latest Syllabus and marking scheme as prescribed by the CBSE is given here. Paper 4 of Solved CBSE Sample Paper for Class 12 Political Science is given below with free PDF download solutions.
Time Allowed: 3 hours
Maximum Marks: 80
General Instructions
- All questions are compulsory.
- Questions nos. 1 to 5 are of 1 mark each. The answer to these questions should not exceed 20 words
- Questions nos. 6 to 10 are of 2 marks each. The answer to these questions should not exceed 40 words
- Questions nos. 11 to 16 are of 4 marks each. The answer to these questions should not exceed 100 words
- Questions nos. 17 to 21 are of 5 marks each. The answer to these questions should not exceed 150 words
- Questions no. 21 is map based question
- Questions nos. 22 to 27 are of 6 marks each. The answer to these questions should not i exceed 150 words
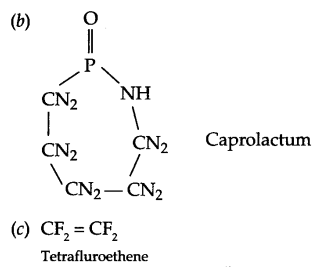
Question 1.
Mention the Second World War period and Korean War period.
Question 2.
What is a capitalist economy?
Question 3.
What is meant by hegemony?
Question 4.
Why was State Reorganisation Commission set up?
Question 5.
Why were the states reorganised on linguistic basis in India in 195 ?
Question 6.
Give the significance of the following dates.
(i) 8 Aug. 19 7
(ii) 25 March 1957.
Question 7.
Mention the features of SAFTA.
Question 8.
What are the Millennium Development Goals?
Question 9.
“India’s experiment with universal adult franchise appeared very bold and risky.” Justify the statement.
Question 10.
What are the key conflicts associated with Orissa (Odisha) reserved iron resources?
Question 11.
Highlight any two/four major objectives of the Prime Minister Nehru’s foreign policy.
Question 12.
Write a note an Arms Race and its effects.
Question 13.
What were the important land reforms started by the Government of India? Why did some of them become unpopular in India?
Question 14.
What is meant by environment? Suggest any two steps for the environmental improvement.
Question 15.
Bring out three differences each between Socialist Party and the Communist Party and between Bharatiya Jana Sangh and Swatantra Party.
Question 16.
What are the various positions on the issue of regional autonomy for Kashmir? Which of these do you think are justifiable? Give reasons for your answer.
Question 17.
Read the following passage carefully and answer the following questions:
The Soviet system, however, became very bureaucratic and authoritarian, making life very difficult for its citizens. Lack of democracy and the absence of freedom of speech stifled people who often expressed their dissent in jokes and cartoons. Most of the institutions of the Soviet state needed reform the one-party system represented by the communist party of the Soviet union had tight control over all institutions and was unaccountable to the people. The party refused to recognise the urge of people in the fifteen different republics that formed the Soviet Union to manage their own affairs including their cultural affairs. Although, on paper, Russia was only one of the fifteen republics then together constituted the USSR in reality Russia dominated everything, and people from other regions felt neglected and often suppressed.
(i) What was Soviet system?
(ii) How many republics formed Soviet Union?
(iii) Which republic dominated in the USSR?
(iv) Why did people become dissatisfied with the rule of Communist Party of Soviet Union?
Question 18.
Read the following passage carefully and answer the questions:
Some people argue that it is strategically more prudent to take advantage of the opportunities that hegemony creates. For instance, raising economic growth rates requires increased trade, technology transfers and investment, which are best acquired by working with rather than against the hegemon. Thus, it is suggested that instead of engaging in activities opposed to hegemonic power, it may be advisable to extract benefits by operating within the hegemonic system. This is called the bandwagon strategy.
(i) What is prudent during a period of hegemony?
(ii) What benefits can be acquired within the hegemonic system?
(iii) What is the bandwagon strategy?
Question 19.
Study the picture given below and answer the questions that follow:

(i) What does the cartoon represent?
(ii) What does the term ‘Tug of war’ refer to?
(iii) Who has been shown on the branches of tree?
Question 20.
Read the passage given below carefully and answer the questions:
Once an emergency is proclaimed, the federal distribution of powers remains practically suspended and all the powers are concentrated in the hands of the union government. Secondly, the government also gets the power to curtail or restrict all or any of the Fundamental Rights during the emergency. From the wording of the provisions of the Constitution, it is clear that an Emergency is seen as an extra¬ordinary condition in which normal democratic politics cannot function. Therefore, special powers are granted to the government.
(i) When was Emergency imposed?
(ii) Who recommended Emergency to be imposed and to whom?
(iii) Mention the implications of Emergency.
Question 21.
Study the following map of European Union and mark the new member countries and older member countries.

(i) Given the significance of the circle of stars for Europe.
(ii) Why are there 12 stars in the circle of the European Union flag?
(ii) Name any two members of the European Union.
Question 22.
How are the external powers influencing bilateral relations in South Asia? Take any one example to illustrate your point.
OR
Critically evaluate the difficulties involved in implementing the suggested reforms to reconstruct the UN.
Question 23.
Looking at the Indian scenario, what type of security has been given priority in India, traditional or non-traditional? What examples could you cite to substantiate the arguments?
OR
What are the economic implications of globalisation? How has globalisation impacted on India with regard to this particular dimension?
Question 24.
Analyse any six consequences of the partition of India in 1947.
OR
What were the major differences in the approach towards development at the time of Independence? Has the debate been resolved?
Question 25.
Write a note on the war and peace with Pakistan.
OR
Discuss the major issue which led to the formal split of the Congress Party in 19 9.
Question 26.
“Emergency was a Blackmark in Indian History”? Comment.
OR
The Bharatiya Kisan Union is a leading organisation highlighting the plight of farmers. What were the issues addressed by it in the nineties and to what extent were they successful?
Question 27.
“The decade of 1980s witnessed tragic turn of events which complicated the problem of Punjab.” Justify the statement in the contest of Operation Blue Star.
OR
Many people think that a two-party system is required for successful democracy. Drawing from India’s experience of last twenty years, write an essay on what advantages the present party system in India has.
Answers
Answer 1.
The Second World War 1939 – 45
Answer 2.
In this economy, land and productive assets are owned and controlled by the capitalists.
Answer 3.
An international system to dominate world by only one superpower.
Answer 4.
It was set up to look into the matter to redraw the boundaries of states.
Answer 5.
States were reorganised on linguistic basis in India in 1956 to maintain unity and integrity of the nation to avoid violence and conflicts among the people.
Answer 6.
- 8 Aug. 1967 — ASEAN was established.
- 25 March 1957 – Six countries signed the treaties of Rome to establish the European Economic Community (EEC) and the European Atomic Energy Community (EURATOM).
Answer 7.
South Asian Free Trade Agreement (SAFTA) was signed by SAARC members in 2004 with the following features:
- Formation of Free Trade Zone for whole South Asia.
- To sustain mutual trade and cooperation among SAARC members.
Answer 8.
The Millennium Development Goals can be categorised as follows:
- Environmental Protection
- Promotion of globalisation
- Anti-terrorism initiatives
- Enhancement and protection of Human Rights.
Answer 9.
Because :
- Country’s vast size and electorates made these elections unusual.
- The year 1952, it was a big test for poor and illiterate country.
- Till then, democracy had been existed only in the prosperous countries mainly in Europe and North America where everyone was almost literate.
Answer 10.
- These iron resources lie in most underdeveloped and predominant tribal districts.
- Tribal population feared that the setting up of industries would mean displacement from their name and livelihood.
- The environmentalist feared to be polluted the environment due to mining and industrial activities.
Answer 11.
- The first objective was to follow NAM, not to join either the military blocs formed by USA or Soviet Union.
- To promote rapid economic development and maintain cordial relations with other nations.
- To prefect the territorial integrity.
- To preserve sovereignty of India and also respecting others sovereignty.
Answer 12.
As all the countries want to protect their military and economic interests to become powerful at international level, a stiff competition emerged among the nations. The powerful and weak both want to become more powerful by stocking arms including nuclear weapons. After the Second World War, it became worse as all the countries including developing nations spent a major portion of their budget on military alliances. The scientists have declared that this blind and unending arms race will harm the food, air and water and ultimately lead to devastation. Money spent on it should be used for welfare purpose.
Answer 13.
To get secure access to land for the poor section of the sociely since independence policies of land reform were implemented to benefit poor and landless poorer section of society. After that various land reforms have been done by the government. Some of them are:
- Abolition of Zamindari System
- Consolidation of small land holdings
- Putting an upper limit or ceiling on the maximum amount of land one can possess.
The main objective of the land reforms was to remove rural poverty and increase agricultural production, but it became unpopular because of the following reasons:
- Some of these land reforms could not be properly implemented because the land owners had both power and political influence.
- These land reforms were not defined into laws and remained only on papers.
Answer 14.
Environment refers to surroundings of a region which can be improved by taking following steps:
- More focus should be on afforestation i.e. planting more trees to maintain ecological balance, prevent soil erosion and enhance water cycle also.
- Eco-friendly industries should be set up as well as industries adherent should be disposed with scientific methods and industries should be established at far away places from populous/residential areas.
Answer 15.
(i) Differences between Socialist party and Communist party:
| Socialist Party | Communist Party |
| (a) This party believed in ideology of democratic socialism. | (a) This party believed in communism. |
| (b) This party criticised capitalism and for establishment of socialistic state. | (b) This party was primarily secular, modern and also authoritarian. |
| (c) This party wanted more radical and egalitarian nature of the Congress. | (c) This party also wanted radical nature of Congress but went through violence to achieve its aims. |
(ii) Defferences between Bharatiya jana Sangh and Swtatantra Party:
| Bharatiya Jana Sangh | Swatantra Party |
| (a) This party emphasised on the ideology of one country, one culture and one nation. | (a) This party emphasised on the free economy and less involvement of government in controlling the economy. |
| (b) This called for a reunity of India and Pakistan in Akhand Bharat. | (b ) It was critical to policy of non-alignment and favoured to have closer relations with the USA. |
| (c) It was a consistant advocate of India to develop nuclear weapons. | (c) It criticised centralised planning nationalisation and one public sector. |
Answer 16.
On the issue of regional autonomy for Kashmir, the following positions are states as:
- Kashmiris were promised to make accession on reference of people after situation created by tribal invasion, becomes normal. But it has not been fulfilled, hence, it generated the demand for “Plebiscite”.
- Sometimes, it was felt that special federal status guaranteed by Article 370 has been eroded practically which led the demand for restoration of autonomy or “Greater State Autonomy”.
- It is felt that democracy, which is practised in rest of India has not been similarly institutionalised in Jammu and Kashmir.
We prefer the first position because ‘Plebiscite’ provides better opportunity to people of J & K to protect and sustain their regional autonomy in a very democratic manner.
Answer 17.
- Soviet system was bureaucratic and authoritarian making life difficult for citizens.
- Soviet system lacked democracy and the freedom of speech of people was also snatched away.
- 15 Republics
- Russia
- Soviet Union had tight control over all institutions.
- Soviet Union was unaccountable to people.
- Soviet Union Refused 15 Republics to manage their own affairs.
Answer 18.
- To take advantage of opportunities that a hegemon creates.
- Increase trade, technology transgers and investment
- To extract benefits by operating within hegemonic system in place of being engaged in the opposed activities.
Answer 19.
- Cartoon represents dominance of the Congress which is being tug by opposition parties to throw the Congress out of power.
- ‘Tug of war’ refers to pulling out the Congress by criticism and mentioning its weaknesses in an honest and justified manner.
- Pt. Jawaharlal Nehru along with his Cabinet Ministers.
Answer 20.
- 25 June 1975.
- The Prime Minister Indira Gandhi recommended to impose Emergency to the President Fakhruddin Ali Ahmad.
- The federal distribution of powes emains practically suspended.
- All the powers ae concentrated in the hands of Union government.
- The government also gets power to restrict all of any of Fundamental Rights during Emergency.
Answer 21.
- The circle of stars stands for harmony and solidarity between the Europeans.
- There are 12 stars in the circle of the European Union flag because, the no. 12 is considered the symbol of unity, perfection and completeness traditionally.
- Lithuania and Poland.
Answer 22.
The external powers influence bilateral relations in South Asia because no region exists in the vacuum. It is influenced by outside powers and events no matter how much it may try to insulate itself from non-regional powers:
- China and the US remain key players in South Asian politics.
- Sino-Indian relations have improved significantly in the last ten years, but China’s strategic partnership with Pakistan remains a major irritant.
- The demands of development and globalisation have brought the two Asian giants closer and their economic ties have multiplied rapidly since 1991.
- The Us enjoys good relations with both India and pakistan and works as a moderator in Indo-Pak relations.
- Economic reforms and liberal economic policies in both the countries have increased the depth of American participation.
- The large South Asian economy remains in the US and the huge size of population and markets of the region give America an added stake in the future of regional security and peace.
OR
In 1992, the UN General Assembly adopted a resolution which reflected three main complaints—
- The Security Council no longer represents contemporary political realities.
- Its decisions reflect only Western values and interests and are dominated by a few powers.
- It lacks equitable representation. In view of these growing demands for the restructuring of the UN, on 1 January, 1997, the UN Secretary General Kofi Annan initiated an inquiry into how the UN should be reformed. How for instance, should new Security Council members be chosen?
In the years since then, the following are just some of the criteria that have been proposed for new permanent and non-permanent members of the Security Council. A new member, it has been suggested, should be a major economic power, a major military power, a substantial contributor to the UN budget, etc. Clearly, each of these criteria has some validity. Governments saw advantages in some criteria and disadvantages in others depending on their interests and aspirations.
It has also been suggested that the veto power of the five permanent members should be abolished. Many perceived the veto to be in conflict with the concept of democracy. But there is also a realisation that the permanent members are unlikely to agree to such a reform.
Answer 23.
India has faced traditional (military) and non-traditional threats to its security that have emerged from within as well as outside its borders.
Its security strategy has four broad components i.e.:
- To strengthen its military capabilities :
- India has been involved in conflict with its neighbours as Pakistan in 1947-48, 1965, 1971 and 1999 and China in 1962.
- In South Asian Region, India is surrounded by nuclear armed countries. Hence, India’s decision to conduct nuclear test in 1998 was justified to safeguard national security.
- India first tested nuclear device in 1974.
- To strengthen international norms and international institutions :
- India’s first Prime Minister Jawaharlal Nehru supported Asian solidarity, disarmament, decolonisation and the UN as a forum to settle down international conflict.
- India took initiatives to bring about a universal and non-discriminatory non¬proliferation regime to enjoy some rights and obligations with respect to weapons of mass destruction.
- It used non-alignment to help to carve out an area of peace outside the blocs.
- India signed Kyoto Protocol in 1997 to be a part of roadmap for reducing the emissions of greenhouse gases to check global warming.
- To meet security challenges within the country :
- Several militant groups from areas such as Nagaland, Mizoram, Punjab, Kashmir have sought to break away from India.
- India makes efforts to preserve national unity by adopting a democratic political system by providing freedom of speech and expression alongwith the right to vote.
- To develop its economy :
- India develops the way to lift vast mass of citizens out of poverty, misery and huge economic inequalities.
- A democratically elected government is supposed to combine economic growth with human development without any demarcation between the rich and the poor.
OR
(A) Economic implications of globalisation
- It involves greater economic flows among various countries.
- It has enhanced trade in commodities among countries.
- The restrictions on the imports and movement of capital have also been reduced.
- This has spread internet and computer related services across national boundaries.
Impact of globalisation on India
- More new jobs have been created in the MNCs like cell phones, fast food etc.
- India is playing a crucial role among developing countries in trade and commerce by making some companies multinational themselves i.e. Tata Motors, Ranbaxy etc.
- Direct Foreign Investment have also been increased.
- It has invited inflow of private foreign capital and export-oriented activities.
Answer 24.
Consequences of the partition of India :
- The year 1947 was the year of one of the largest, most abrupt, unplanned and tragic transfer of population that Indian history was known. In the name of religion, people of a community killed and maimed people of the other community. Cities like Lahore, Calcutta (Kolkata) and Amritsar were titled as communal zones.
- Muslims would avoid going into areas where mainly Hindus and Sikhs lived. Similarly, the Hindus and Sikhs stayed away from Muslim areas.
- People went through immense sufferings because they were forced to abandon their homes and move across borders. Minorities on both sides of the border fled their homes and often secured temporary shelter in ‘refugee camps’. They often found helpless local police and administration helpless in what was till recently their own country. They travelled to the other side of the new border by all sorts of means, often by foot. Even during this journey they were often attacked, killed or raped. Thousands of women were abducted on both sides of the border. They were made to convert to the religion of the abductor and were forced into marriage. In many cases, women were killed by their own family members to preserve the ‘family honour’. Many children were separated from their parents.
- Those who did manage to cross the border found that they had no home. For lakhs of these ‘refugees’ the country’s freedom meant life in refugee camps, for a long time,
- While recounting the trauma of partition, they have often used the phrase that the survivors themselves used to describe partition—as a division of hearts.
- The partition was not merely a division of properties, liabilities and assets, or a political division of the country and the administrative apparatus. The employees of the government and the railways were also divided. Partition forced about 80 lakh people to migrate across the new border. About 5 to 10 lakhs people were killed in partition-related violence. However, beyond the administrative concerns and financial strains, the partition posed another deeper issue, the leaders of the Indian national struggle did not accept the two-nation theory. And yet, partition on religious had taken place.
OR
At the time of Independence, development was about becoming more like the industrialised countries of the West, to be involved with the break down of traditional social structure as well as rise of capitalism and liberalism.
- Modernisation referred to growth, material progress and scientific rationality.
- India had two models of modern development at the time of independence into considerations to be adopted i.e. the liberal capitalist model like Europe and the US and the socialist model like the USSR.
- A debate had been occurred regarding adoption of model of development as communists, socialists and Pt. Jawaharlal Nehru supported the socialist model to reflect a broad consensus to be developed during national movement,
- Above mentioned intentions cleared that the government made the priority to poverty
alleviation alongwith social and economic redistribution. - At the same time, these leaders differed and debated:
- Industrialisation should be the preferred path or
- Agricultural development should take place, or
- Rural poverty should be alleviated.
Answer 25.
Indo-Pak conflict started just after partition over the dispute on Kashmir. A proxy war broke, out between the Indian and Pakistani armies in Kashmir during 1947 itself. But this did not turn into a full war. Both the governments of India and Pakistan worked together to restore the women abducted during partition to their original families. A long¬term dispute about the sharing of river waters was resolved through mediation by the World Bank. The India-Pakistan Indus Waters Treaty was signed by Nehru and General Ayub Khan in 1960.„Despite all ups and downs in the Indo-Pak relations, this treaty has worked well.
In April 1965, Pakistan launched armed attacks in the Rann of Kutch area of Gujarat. This was followed by a bigger offensive in Jammu and Kashmir in August-September. Pakistan did not get support from the local people there. In order to ease the pressure on the Kashmir front, Indian Prime Minister Lai Bahadur Shastri ordered Indian troops to launch a counter-offensive on the Punjab border. In a fierce battle, the Indian army reached close to Lahore.
The hostilities came to an end with the UN intervention. Later, Shastri and Pakistan’s General Ayub Khan signed the Tashkent Agreement, brokered by the Soviet Union in January 1966.
OR
The formal split in Congress took place in 1969 on the issue of nomination of the candidate during presidential elections:
- Despite, Indira Gandhi’s reservations, the Syndicate nominated Neelam Sanjeeva Reddy, as the official Congress candidate for ensuing Presidential elections.
- Indira Gandhi retaliated the situation by encouraging V.V. Giri, the then Vice-President, to be nominated as an independent candidate.
- During election, the then Congress President S. Nijalingappa issued a whip asking all Congress MPs. MLAs to vote for N. Sanjeeva Reddy.
- On the other hand, after silently supporting V.V. Giri, the Prime Minister Indira Gandhi openly called for a conscience vote to vote the way they want’.
- Elections went in favour of V.V. Giri due to this diplomatic effort and N. Sanjeeva Reddy was defeated.
- The defeat of N. Sanjeeva Reddy, the formal Congress candidate, formalised the split of party into two:
- Congress (0), i.e. organisation led by syndicate, known as Old Congress.
- Congress (R) i.e. requisitionists led by Indira Gandhi, known as New Congress.
Answer 26.
- Emergency was declared on the ground of internal disturbances on 25 June 1975 to invoke Article 352 of the Constitution.
- The Prime Minister Indira Gandhi recommended to impose Emergency to the President Fakhruddin Ali Ahmad.
- Emergency was one of the most controversial episodes which possessed different virus regarding imposition of Emergency.
- Emergency practically suspended the democratic functioning.
- ‘Shah Commission’ exposed many excesses committed during emergency.
- Emergency highlighted some hidden matters over constitutional battle between the Parliament and judiciary.
- Tensions or conflicts had been arisen between institution based democracy and popular participation of people.
OR
Bharatiya Kisan Union (BKU) is an organisation of farmers from the western UP and Haryana regions. It is one of the leading farmers’ movements to protest against the policies of process of liberalisation of Indian economy. The Meerut agitation of farmers was a great show of rural farmers and cultivators.
Issues addressed by BKU :
- Higher government floor prices for sugarcane and wheat.
- Guaranteed supply of electricity at reasonable rates.
- To wave off repayments due on loan to farmers.
- To provide government pension to farmers.
- Abolition of restrictions on the inter-state movement of farm produce.
Highlighted the plight of farmers :
- BKU conducted rallies, demonstrations, and Jail Bharo agitations.
- These protests involved thousands of farmers-sometimes over a lakh-from various villages in western UP and adjoining regions.
- BKU operated as a pressure group in politics with its strength of sheer members.
Extent of Success :
- BKU became the most successful social movements. ‘
- It sustained for a long time due to clan networks among its members.
- These networks mobilised funds, resources and activities of BKU.
- An outcome of political bargaining powers by its members.
- BKU farmers dominated regional electoral politics also.
Answer 27.
The leadership of Akali movement passed from the moderate Akalis to the extremist elements and took the form of armed insurgency. These militants made their headquarters inside the Sikh holy shrine, the Golden Temple in Amritsar, and turned it into an armed fortress. In June 1984, the Government of India carried out ‘Operation Blue Star’ code name for army action in the Golden Temple.
In this operation, the government could successful flush out the militants, but it also damaged the historic temple and deeply hurt the sentiments of the Sikhs. A large proportion of Sikhs in India and abroad saw the military operation as an attack on their faith and this gave further impetus to militant and extremist groups.
More tragic turn of events complicated the Punjab problem further. On 31 October 1984, the whole nation was in mourning when Indira Gandhi was assassinated by her bodyguards outside her residence. Both the assassins were Sikhs and wanted to take revenge for Operation Blue Star. While the entire country was shocked by this development, in Delhi
and in many parts of northern India violence broke out against the Sikh community. In this violence, hundreds of Sikhs were killed in other parts of the country. Many Sikh families lost their male members and thus suffered great emotional and heavy financial loss.
OR
In the first decade of electoral politics, India did not have a recognised opposition party. But some of vibrant and diverse opposition parties had come into being even before the first General Election of 1952 as the non-Congress parties. Hence, the roots of almost all the non-Congress parties of today can be traced to one or the other of the opposition parties of 1950s.
All these opposition parties gained only a representation, still their presence played a crucial role in maintaining democratic character of system. Hence due to following reasons two party system is required for successful democracy:
- Within two party systems, the opposition party offers a sustained and principled criticism of policies and practices of ruling party keeping it under a strict check.
- By keeping democratic political alternative alive, these parties prevented the resentment with the system from turning anti-democratic.
On the basis of above mentioned features it is justifiable to have a two party system which have following advantages:
- India has arrived at more competitive politics.
- Political parties act within the spheres of consensus.
- New forms, vision, pathways of development have been identified.
- Issues like poverty, displacement, minimum wages, livelihood and social security are being put on political agenda.
- Issues of justice and democracy are being voiced by various classes, castes and regions to remind states its responsibility.
We hope the CBSE Sample Papers for Class 12 Political Science Paper 4 help you. If you have any query regarding CBSE Sample Papers for Class 12 Political Science Paper 4, drop a comment below and we will get back to you at the earliest.
![]()
![]()

![]()