Here we are providing Class 11 Biology Important Extra Questions and Answers Chapter 8 Cell: The Unit of Life. Important Questions for Class 11 Biology are the best resource for students which helps in Class 11 board exams.
Class 11 Biology Chapter 8 Important Extra Questions Cell: The Unit of Life
Cell: The Unit of Life Important Extra Questions Very Short Answer Type
Question 1. Who gave the term chromosome?
Answer:
Waldayer.
Question 2.
Define a cell coat.
Answer:
A clear layer of oligosaccharide outside the cell membrane in some animal cells.
Question 3.
Where is dynein present?
Answer:
In microtubules of flagella.
Question 4.
Define sarcoplasmic reticulum.
Answer:
It is defined as the ER found in striated muscles.
Question 5.
What are pili?
Answer:
Pili are elongated, tubular structure in Gram-ve bacteria.
Question 6.
What are fimbriae?
Answer:
Fimbriae are small, bristle-like fibers sprouting out of the cell in bacteria.
Question 7.
Name the organelle of the cell called ‘Suicidal-bag’?
Answer:
Lysosomes are called suicidal bags of the cell.
Question 8.
Define cytoplasm.
Answer:
The cytoplasm is a jelly fluid of protoplasm composed of inorganic and organic matters containing many organelles like ribosomes, ER, vacuole, etc.
Question 9.
Define plasmodesmata.
Answer:
Plasmodesmata are connections between two cell walls that are interrupted by small pores having fine threads of cytoplasm.
Question 10.
What are the three parts of a flagellum?
Answer:
- filament
- hook and
- basal body.
Question 11.
What is an asymmetric karyotype?
Answer:
A karyotype that shows the larger size, the difference between smaller and larger chromosome of the set, and have few metacentric chromosomes.
Question 12.
What is the origin of the Golgi complex?
Answer:
Smooth endoplasmic reticulum.
Question 13.
What are lysosomal enzymes?
Answer:
Hydrolases.
Question 14.
What are tunnel proteins?
Answer:
Integral proteins of plasma membrane functioning as channels.
Question 15.
Which organelle is called the “engine of the cell”?
Answer:
Ribosomes where protein synthesis occurs.
Question 16.
Define symplasm.?
Answer:
It is a plasmodesma that helps to maintain the continuity of living matter and cytoplasm.
Question 17.
What is desmotubule?
Answer:
A fine cytoplasmic canal lined by a plasma membrane and has ER.
Question 18.
What are permeases?
Answer:
Enzymes facilitate the entry of substances through the plasma membrane.
Question 19.
Who gave the unit membrane concept?
Answer:
Robertson.
Question 20.
Expand PPLO.
Answer:
Pleuro Pneumonia likes organisms.
Question 21.
What holds the ribosome together in a polyribosome?
Answer:
The mRNA chain.
Question 22.
Who discovered the cell?
Answer:
The cell was discovered by Robert Hooke.
Question 23.
What is totipotency?
Answer:
The capacity of every plant cell to develop into a whole plant is totipotency.
Question 24.
Who discovered the nucleus?
Answer:
Nucleus was discovered by Robert Brown in the cells of roots of orchids.
Question 25.
What is tonoplast?
Answer:
Tonoplast is the membrane around vacuole.
Question 26.
What are receptor molecules?
Answer:
Receptor molecules are specific proteins in the cell membrane that enter into the cells like hormones.
Question 27.
What is absent in erythrocytes?
Answer:
Nucleus, Aerobic respiration, DNA.
Question 28.
Who proposed the cell theory.
Answer:
Cell theory was proposed by M.J. Schleiden and Theodore Schwann.
Question 29.
Name two types of cells that retain their mitotic ability but seldom divide.
Answer:
Liver and muscle cells.
Question 30.
Who concluded, “Cells are the ultimate units forming the structure of all plant tissues”?
Answer:
Mathias Jacob Schleiden, a German botanist concluded that the cells were the ultimate units forming the structure of all plant tissues.
Question 31.
What is the contribution of Leeuwenhoek to cell biology?
Answer:
The invention of the microscope.
Question 32.
Name the site for protein synthesis in the cell.
Answer:
Ribosome.
Question 33.
What is differentiation?
Answer:
The process by which cells lose their specialization is called differentiation.
Question 34.
Name some unicellular organisms.
Answer:
Amoeba, Paramecium, Euglena and Acetabularia.
Question 35.
Name two organelles, other than the nucleus, which contain DNA.
Answer:
Answer:
Mitochondria or chloroplast.
Cell: The Unit of Life Important Extra Questions Short Answer Type
Question 1.
Give the fundamental similarities in all cells:
Answer:
Fundamental similarities in all cells are:
- Hereditary characters are transmitted through nucleic acids.
- The basic structure of membranes of all cell organelles is the same.
- Method of aerobic respiration.
- Mechanism of synthesis of nucleic acids and proteins within the cells.
Question 2.
What are Cytoskeletal Structures?
Answer:
Cytoskeletal Structures: The ability of eukaryotic cells to adopt different types of shapes and to perform directed movement depends on the cytoskeleton.
There are three principal types of protein filaments
- Microfilaments,
- Microtubules, and
- Intermediate filaments.
These constitute the cytoskeleton. The microfilaments are 8 nm in diameter, either scattered or organized into the network or parallel arrays within the matrix. They play a major role in cell motion (changes in shape). Such cellular movements associated with the microfibres are movements of pigment granules, amoeboid movements, and protoplasmic streaming. These microfilaments consist of actin-like proteins.
Question 3.
What are the main functions of the cell wall?
Answer:
The main functions of the cell wall are:
- It gives a definite shape to the cell and protects the internal organelles.
- It provides a framework and lends support to the plasma membrane.
- It prevents the cell from desiccation.
- It counteracts physically the osmotic pressure produced by the cell contents.
- It helps in the transport of materials and metabolites in and out of the cell.
Question 4.
List the functions of Golgi bodies.
Answer:
The functions of Golgi bodies are:
- Storage, condensation, and packaging of the material.
- Several enzymes are localized in Golgi bodies.
- During spermatogenesis, Golgi apparatus form the euro some.
- Mucilage and gums are secreted in plant cells due to the action of the Golgi apparatus.
Question 5.
Name different types of the endoplasmic reticulum.
Answer:
There are two types of EM.
- Smooth ER (Agranular) and
- Rough ER.
Question 6.
Describe the functions of the three organelles, viz Golgi bodies, chloroplasts, and mitochondria.
Answer:
(a) Functions of Golgi bodies:
- Carbohydrate synthesis of mucopolysaccharides
- Formation of acrosome
- Formation of the lysosome.
- Formation of the plasma membrane.
- Formation of the cell wall.
- Absorption of compounds.
- Production of hormones.
- Formation of pigments.
- Yolk deposition.
(b) Functions of chloroplast:
- Their main function is to trap the sun’s energy and to convert it into the chemical energy of food by photosynthesis.
- Storage of starch,
- Chloroplasts in fruits and flowers change into chromoplasts.
(c) Functions of Mitochondria:
- Powerhouses the cell and stores energy as ATP.
- Several respiratory enzymes are found in mitochondria.
- DNA is also contained in mitochondria.
- They regulate the concentration of calcium ions in the cells.
Question 7.
Describe functions of flagella and cilia.
Answer:
Functions of flagella and cilia:
- Flagella and cilia help the organisms in movement and loco¬motion.
- They help the organism to swim in the water.
- Social and flagella are associated with the motility of cells.
Question 8.
Distinguish between:
(a) Microtubules and microfilaments.
Answer:
Differences between Microtubules and Microfilaments:
| Points | Microfilaments | Microtubules |
| (1) Structure | (1) Actin is the main component of microfilament and so they are contractile. | (1) (α) and (β) tubulin proteins are the main components of the microtubules so they are non-contractile. |
| (2) Diameter | (2) 5 – 6 nm | (2) 25 nm |
| (3) Sub-units | (3) Absent | (3) 13 protofilaments from a microtubule |
(b) Primary wall and secondary wail.
Answer:
Difference between Primary and Secondary cell walls:
| Character | Primary cell wall | Secondary cell wall |
| (1) Location | (1) Primary cell wall is found in plant cells only. | (1) Secondary cell wall is found in nature and non-dividing cells. |
| (2) Thickness | (2) 1 – 3 pm | (2) 5 – 10 pm |
| (3) Growth | (3) Growth internal | (3) Growth is accretionary. |
| (4) Chemical Structure | (4) It is made of pectin cellulose or hemicellulose | (4) It is made of cellulose, hemicellulose suberin, cutin, or lignin. |
(c) Leucoplast and chromoplast.
Answer:
Differences between Leueoplast and Chromoplast:
| Leueoplast | Chromoplast |
| 1. Colourless plastids devoid of any pigment. | 1. May contain pigments other than chlorophyll. |
| 2. They have the capacity to develop pigment when needed. Leucoplasts are of three types- amyloplast, proteinoplast, and elaioplast. | 2. They synthesize and store other pigments such as carotenoids. |
Question 9.
Describe the ultrastructure and functions of
(a) nucleus
Answer:
Ultrastructure and functions of Nucleus: Electron microscopic studies reveal that the nucleus is bounded by two membranes, which make the nuclear envelope. The outer and inner membranes are separated by a narrow space, perinuclear space.
The outer membrane remains in continuation with the endoplasmic reticulum (ER) and the inner one surrounds the nuclear contents. At some points, the nuclear envelope is interrupted by the presence of small structures called nuclear pores.
These pores are enclosed by circular pores. These pores are enclosed by circular structures called the annuli. The pores and annuli unite to form the pore complex. These pores help in the exchange of materials between nucleoplasm and cytoplasm. Nuclear membrane disappears during cell.-division. It reappears during nuclear recognization in the telophase stage.
The nucleoplasm contains chromatin and nucleolus The nucleolus is a rounded structure, it is not separated from the rest to the nucleoplasm by a membrane. It is associated with a specific nucleolar organizing region (NOR) of some chromosomes. The nucleolus is the “site for ribosomal RNA synthesis.” The cells which remain engaged in protein synthesis have larger and more numerous nucleoplasm their nucleoplasm.
(b) mitochondrion and
Answer:
Ultrastructure of Mitochondria: They are spherical or elongated or rod-like cell organelle. They are known as the “powerhouse of the cell.” Mitochondria was fust discovered by Hofmeister (1851) in the cells of a pteridophyte. Equisetum ( 1851). They were named ‘mitochondrion’ by Benda (1897).
Mitochondria is a double membranous organelle, the outer membrane is smooth while the space between the two membranes is called the outer chamber while the space surrounded by the inner membrane is an inner chamber that is filled with homogenous fluid.
The inner membrane has a large number of F particles or Oxysome. These are sites for oxidative mitochondrial phosphorylation. Each Oxysome has a head, stalk, and base. The number, of elementary particles in mitochondria on maybe 104-105.’ The head of F particle contains ATP’ ( synthetase enzyme hence they are said to be ATP profiles.
(c) plastid.
Answer:
Functions of Mitochondria:
Ultrastructure of plastid: Plastids are green-colored cell organelles in the form of plastids. They are bounded by two membranes about 300 A in total thickness. Each membrane is about 40 to 60 A’ thick. Both the membranes are separated by a clear space of about 25 – 27 A.
The inner membrane is very intricately elaborated to form a system of lamellae. Internally the chloroplasts are divisible into two parts, stroma – (colorless; ground substance) and the membrane system (made of closed flattened sacs called thylakoids).
The thylakoids are closely packed in certain areas. They appear as piles of coins placed one above the other. These structures are called grana. As many as 40 to 60 grana may be present in a single chloroplast and eish granum may contain 2 to 100 coin-like thylakoids.
Thylakoids can be seen in a variety of configurations in different species of plants. The arrangement can be in the form of simple parallel sacs running lengthwise, 4, or maybe in a complex interconnecting network of the sacs. The chloroplasts invariably have the same starch granules which often accumulate near a special region known as pyrenoid in algae.
Question 10.
Describe the fluid mosaic model of the plasma membrane.
Answer:
Structure of plasma membrane: Fluid mosaic model of the plasma membrane was suggested by, S, Singer, and G. Nicholson in the 1970s.
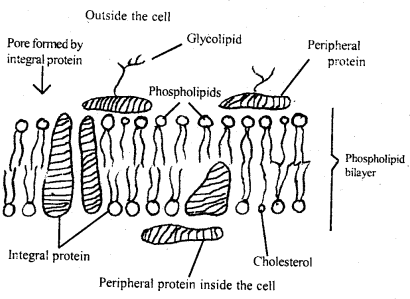
Fluid-mosaic model of membrane
According to this model, the lipids and proteins are arranged in a mosaic fashion. The matrix is a highly viscous fluid of two layers of phospholipids having two types of protein molecules – extrinsic and intrinsic proteins.
The phospholipid layer is bimolecular and their hydrophilic ends are pointed towards the top and bottom respectively. Peripheral (extrinsic) proteins are superficially arranged on either side and can be easily separated. They have enzymatic properties and also make membranes selectively permeable.
Integral (intrinsic) proteins are tightly held in place by strong hydrophilic or hydrophobic interactions or both and are difficult to remove from the membranes.
Question 11.
What is a cell envelope? Describe its chemical nature.
Answer:
Cell envelop and its chemical nature:
Cell envelope: The bacterial cells have a chemically complex cell envelope. The layers of cell envelope stack upon one another and are bonded together tightly.
Three basic layers are identified
- The outermost glycocalyx
- Cell wall and
- The cell membrane (plasma membrane).
Each layer of the envelope performs a specific function. They act as a single protective unit as a whole. The cell envelope accounts for about 10 – 50% of the cell volume.
The glycocalyx is the outermost layer. It has a coating of macromolecules. These macromolecules protect cells. They help in adhesion. It differs in thickness and chemical composition in different bacteria. Some prokaryotes have a loose sheath called the slime layer.
This slimy layer protects the cells from loss of water and nutrients. In some bacteria, there may be a thick covering called a capsule. The capsule and slime layer consists of polysaccharides and proteins. The capsule is responsible for the gummy and sticky character of the cell. This layer may be highly specific and immunogenic in bacteria:
Question 12.
Give the difference between cell walls of Gram-positive and Gram-negative bacteria?
Answer:
| Gram-positive bacteria | Gram-negative bacteria |
| 1. Their cell wall is only single-layered and 100-200 A thick. | 1. Their cell wall consists of two layers and is 70-120 A in thickness. |
| 2. They are stained by gram stain. | 2. They are not stained by gram stain. |
| 3. They do not have pili. | 3. They have pili |
| 4. In gram-positive bacteria they are mesosomes. | 4. In gram-negative bacteria they are not present. They are poorly developed. |
Question 13.
What are the cell inclusions in a prokaryotic cell?
Answer:
Cell inclusions in prokaryotic cells are granules or inclusion bodies. They lie freely in the cytoplasm. For example, phosphate granule; glycogen granules, sulfur granules, gas vacuole, poly-(ii) hydroxybutyrate. There may be metachromatic granules.
Question 14.
What is the structure of the ribosome?
Answer:
Ribosome Palade (1855) coined the term ribosome and he dis-covered them in animal cells. The size of the ribosome varies from 150 A0 to 250 A° in diameter. The size of the ribosome can be determined by the speed with which they sediment in a centrifugal field. The sedimenting speed measured in a unit called Swedberg unit S, The size of ribosomes present in higher animal cells is about 80S whereas the size in bacteria is about 70S.
Structure of ribosomes: Each ribosome is made up of two sub-units. For example, the ribosome of SOS has the 60S and 40S sub-units whereas 70S ribosomes have 50S and 30S subunits. These sub-units are further formed of smaller sub-units. The ribosomes are formed in the nucleolus.
Each sub-unit of ribosomes consists of about an equal amount of RNA protein. The ribosomal proteins are formed somewhere in the cytoplasm but then it migrates to the nucleoli. The ribosomal RNA is formed by ribosomal genomes found in the nuclear DNA. The ribosomal protein joins the so formed ribosomal RNA and results in, the formation of ribosomes.
Question 15.
Mention the differences between Pilus and Fimbriae.
Answer:
The differences between Pilus and Fimbriae:
| Pilus | Fimbriae |
| 1. The pili are elongated and tribal structure. | 1. The fimbriae are small bristle-like fibers. |
| 2. Pili is made up of a protein called pilin. | 2. Fimbriae are composed of helically arranged protein sub-units. |
| 3. Pili are involved in the mating process. | 3. Fimbriate attach bacteria to a solid surface. |
Question 16.
Give point is the justification of the statement “cell is the basic unit of life.”
Answer:
The cell-The basic unit of life: All living organisms are composed of small, tiny structures or compartments i.e. cells. These cells are called the building blocks of life.
The cells in the true sense are considered as the basic unit of life because all the life processes i.e., metabolism, responsiveness, reproduction are carried out by the cells. The cell is the seat of all metabolic (anabolic and catabolic) processes. Respiration, nutrition release of energy for the body are earned out within the cells only. Even the animals and plants (organisms) reproduce because the cell reproduces individually. Growth occurs because cells grow and multiply.
Let us take the example of Amoeba, a unicellular organism. In Amoeba all the life processes are performed within the boundaries of the single cell. This is true for all other multicellular organisms. The only difference, in the multicellular organisms, is the body of these organisms is made up of many cells. In these organisms, the cells do not behave independently but get organized into tissues.
Each tissue is specialized to perform specific functions. Different tissues then get, organized into organs that perform certain specific functions. Different organs are finally organized to form organ systems. Now it must be very clear that the basic structure of tissues, organs, and organ system are the cells only. These tissues, organs, and organ systems of the .organisms work because the cell works.
On account of the above, it can be said that cells are the structural and functional unit of the living being, hence it is the basic unit of life.
Question 17.
“The function of an organism is the result of the sum total of activities and interactions of the constituent cells.” Comment.
Answer:
The cells are building blocks of bodies. They are the functional units of bodies -Schleiden and Schwann observed that all animals and ‘ plants are composed of cells. The cells make tissues, tissues make, organs and organs make organ system. Organ systems are interrelated to perform a specific function of that system. In the body of an individual, there are different types of cells that serve different types of functions.
The activities of an organism are sum total of co-ordinate activities and interactions of the constituent cells, the cells in the body have the same hereditary material. All new cells of an organism develop from the pre-existing cells, so all cells have DNA hereditary material or RNA which passes from one generation to other by division.
So each cell has the same genetic information and it has the potential to give rise to a new individual. This property of the cell is called totipotency.
Question 18.
Multicellular organisms have better survival chances than their unicellular counterparts. Why?
Answer:
Division of labor: Some organisms are made up of just one cell (Amoeba, Paramecium, Chlamydomonas, etc.) These are termed unicellular organisms. In these organisms, all the vital life activities are performed within a single cell. On the other hand, most organisms are formed by many cells.
The size of these organisms is also big. In these multicellular organisms, all the body cells do not perform all the vital activities of life. The multicellular organisms have an advantage over unicellular organisms because in these organisms cells play a more specialized role in life activities.
For example in multicellular animals some cells of the body perform the function of movement (muscle cells), some perform the function of digestion or respiration or the removal of the wastes from the body. A group of specific types of cells performs only some limited functions.
Similarly in higher plants, some of the cells perform protective functions (epidermal cells) some cells perform the function of transport of water, mineral, and food substance in the body (xylem and phloem).
These cells would perform other functions except for which they are specialized. The group of similar cells performing similar functions is termed tissues. This system is very advantageous to multicellular organisms. Because the work has been divided by the cells of the body. This is called the division of labor. Due to this division of labor among the cells all the vital activities in the body of an organism function in a coordinated way.
Question 19.
Write a note on the structure of the nucleus.
Answer:
Structure of Nucleus: The nucleus is one of the most important components of the cell. It is, therefore, called the control center of the cell as it controls the various metabolic activities of the cell. The nucleus is situated in the cytoplasm of the cell. Usually, it is round but many different shaped nuclei can be seen in some cells.
It is surrounded by two porous membranes called nuclear membranes which remain continuous with the ER. Within the nuclear membrane is present a liquid substance called nucleoplasm. The nucleoplasm contains chromatin material of two types heterochromatin and euchromatin.
Question 20.
Write a brief note on the bacterial cell wall.
Answer:
The cell wall of Bacteria: It is formed of murein or peptidoglycan. It consists of polysaccharides cross-linked with short amino acid chains. In Gram, -ve bacteria covering is formed of lipopolysaccharide and present around cell wall. It may provide. specific adhesion properties to these cells. It determines the shape of the cell.
Question 21.
“The function of an organism is the result of the sum total of activities and interactions of the constituent cells.” Comment
Answer:
The-cells are building blocks of bodies. They are the functional units of bodies -Schleiden and Schwann observed that all animals and plants are composed of cells. The cells make tissues, tissues make organs and organs make organ systems. Organ systems are interrelated to perform a specific function of that system.
In the body of an individual, there are different types of cells that serve different types of functions. The activities of an organism are sum total of co-ordinate activities and interactions of the constituent cells. The cells in the body have the same hereditary material All new cells of an organism develop from the pre-existing cells, so all cells have DNA hereditary material or RNA which passes from one generation to another by division.
So each cell has the same genetic information and it has the potential to give rise to a new individual. This property of the cell is called totipotency.
Question 22.
How is the multicellular organization of the body more advanced than unicellular organization?
Answer:
The multicellular organization is more advanced than the unicellular organization as:
- Division of labor among cells increases efficiency. In a multicellular organism, cells are differentiated. Specialized cells perform specific functions. Their varied type of cells is more efficient than a single-celled organism.
- Increase in survival capacity. In multicellular organisms, the death of few cells does affect the whole organism.
Question 23.
Give four examples of specific functions performed by varied cells.
Answer:
- Nerve cells are specialized to transmit nerve impulses.
- Muscle cells are specialized for contraction
- Cells of the pancreas for secretion of insulin.
- Red blood cells for the transport of oxygen,
Question 24.
“Cell theory has shortcomings”. Justify.
Answer:
There are some shortcomings of cell theory which are given below.
- Schleiden and Schwann were not the first scientists to prove that living beings are made up of cells.
- Rudolf Virchow stated that “all-new cells arise from the pre-existing cells”.
- Now it is able to explain that all cells contain hereditary material which passes from one generation to the next by cell division. It ensures the transfer of characters from parents to their offsprings.
Question 25.
Why does the efficiency of a cell decrease with an increase in size in a unicellular organism?
Answer:
With the increase in size, volume increases but surface area exposed to the environment does not increase correspondingly. This limits the exchange of information and materials through the surface. As a result efficiency of the cell as an autonomous unit decreases.
Question 26.
The cell is “an open dynamic system” discuss.
Answer:
The cell is an open system because it allows the entry and exit of matter and energy. It takes up food, oxygen, water, and salts for its substance, growth, and division. The cell also takes up energy from- food and operates the metabolic processes. It gives out waste products, secretions, and energy.
Question 27.
On what basis can we consider the cell as an autonomous unit?
Answer:
The cell can be considered as an autonomous unit because:
- Each cell-carries out all fundamental biological processes independently.
- Each cell oxidizes food material and utilizes that energy and some nutrient molecules to synthesize complex molecules.
- The cell uses these molecules to build new structures and to replace worn-out cells.
- The cell respires and exchanges gases with the environment.
- It reproduces to form new cells with similar hereditary characters,
- It also maintains an internal physiochemical environment.
Question 28.
Distinguish between Extrinsic and intrinsic flow of information
Answer:
| Extrinsic | Intrinsic |
| 1. Flow of genetic, information within the cell. | 1. Flow of functional information from outside the cell, |
| 2. Regulates all activities of the cell. | 2. Regulates some activities of the cell. |
| 3. Information flows in the form of nucleic acids. | 3. Information flows in the form of protein or other types of molecules. |
Cell: The Unit of Life Important Extra Questions Long Answer Type
Question 1.
What is the role of the plasma membrane in the compartmentalization of the cell?
Answer:
Every cell is enclosed on all sides by the distinct covering called the plasma membrane.
- It maintains the individuality of the cell by preventing the mixing of cell contents with extracellular materials.
- The cell is not a sealed compartment and the exchange of materials is allowed by the cell membrane in a selective and regulated manner.
- In animals, the cell membrane has on its surface certain chemical that can recognize cells of the same kind. This helps the cells to aggregate and defend themselves against microbes.
- The cell membrane also receives messages from outside and passes them to adjacent cells.
- The cell membrane selectively allows the passage of certain molecules and ions from the seawater to enter the cells of organisms living in the sea.
Question 2.
Compare a plant cell with an animal cell.
Answer:
| Plant cell | Animal cell |
| 1. Plant cells form all the amino acids, coenzymes, and vitamins. | 1. Animal cells cannot form all the amino acids, coenzymes, and vitamins. |
| 2. A plant cell consists of a cellulose cell wall surrounding the plasma membrane | 2. The cell wall is absent. The limiting membrane of the cell is the plasma membrane. |
| 3. On plasmolyzing, the cells do not burst due to the presence of a cell wall. | 3. On plasmolyzing animal cells burst due to the absence of a cell wall. |
| 4. A plant cell consists of plastids in general. | 4. In an animal cell, the plastids are generally absent. |
| 5. Plant cells consist Of large vacuoles. | 5. Vacuoles are absent. |
| 6. Crystal may be present in plant cells. | 6. Crystals are usually absent in animal cells. |
| 7. Centriole is absent in plant cells. | 7. Centriole is present though absent in invertebrates. |
| 8. Mitochondria are fewer. | 8. Mitochondria are numerous. |
Question 3.
The cells of unicellular organisms are usually spherical whereas those of multicellular tend to be many-sided. Why?
Answer:
- It is true that the cells of unicellular organisms tend to be spherical. It is because of the following reasons.
- Surface tension: Surface tension shapes the spherical way as in the case in air-borne soap bubbles.
- The free-floating cells with thin membranes tend to be spherical as it is the most economical shape that can confine a given mass of protoplasm.
However, the shape and the size of the cell depend upon the place where they are present and the functions they have to perform. In multicellular animals, the cells tend to become faceted as they come in contact with each other in the same way as the spherical soap bubbles become flattened when they are jammed together in a small space.
This phenomenon can be best seen in the early stages of the development of an embryo. The cell mass still remains spherical for some time but when the cells multiply the shape changes because of adjusting themselves to the available space. This is also true in plants, they have in addition cellulose also.
But the most important aspect of cell shape is the functions each cell has to perform.
Question 4.
Discuss how the method of science is reflected in the formulation t of cell theory?
Answer:
Methods of science and cell theory: Scientific method of solving a problem is a realistic approach and it helps us in finding out the truth.
The scientist after making observations of many samples proposes a hypothesis that is subject to be tested before it is taken as a theory.
1. In the case of cell theory Theodor Schwann after examining many types of tissues found that all these have cells and the cells have a nucleus and cytoplasm universally present and then after comparing its structure to that of plant cells proposed a hypothesis that bodies of animals and plants are composed of cells and products of cells.
2. This hypothesis was later confirmed by Schleiden who examined a large variety of plant tissues and found all of them to be composed of cells. Thus the hypothesis of Schwann become a theory of Schleiden and Schwann.
Further, as the scientific method says a theory can be changed, modified, or discarded on the basis of new discoveries. The cells theory of Schleiden and Schwann was modified by Rudolf Virchow who was the first to explain that, “cells divide and all-new cells must come from pre-existing cells.”
Thus we can say that method of science is fully reflected in the formulation. of the cell theory.
Question 5.
If, as the second law of thermodynamics states, “the free energy in any system tends to decrease”, how is it that the earth maintains so many living organisms, each in a highly organized, high free energy state?
Answer:
- To maintain the organization of living organisms every system of energy, try to reduce entropy. Entropy is the degree of randomness. If the system is left on its own it increases in entropy.
- Earth receives a continuous supply of energy from the sun in the form of photons of light.
- 0.2 to 1% of the solar energy received by the earth enters the biosphere in the form of chemical energy through the process of photosynthesis. Heterotrophs depend upon autotrophs for food.
- Flows of energy take place from photosynthesizers to heterotrophs „
forming food chains and food webs. - Approximately 10% of the energy is conserved at each trophic level. This is how earth maintains so many living organisms in a highly organized state.
Question 6.
Distinguish between prokaryotes and eukaryotes.
Answer:
| Features | Prokaryotes | Eukaryotes |
| 1. Nucleus | Not enclosed membrane. | Enclosed is the nuclear membrane. |
| 2. Cell wall | Present in all except smallest prokaryote, when present contain muramic acid. | Variable, if present does no contain muramic acid. |
| 3. Endoplas mic reticulum | Absent | Universally present. |
| 4. Organelles | Membrane-bound organelles absent. | Present. |
| 5. Ribosomes | Present freely in the cytoplasm
Only of 70S type with | Present freely in cytoplasm or associated E.R. (80S type). |
| 6. Centrioles | Absent | Present in animal cells |
| 7. Size range | 100-2000 nm | 10,000-100,000 nm. |
Question 7.
Explain the structure and function of mitochondria.
Answer:
Structure of Mitochondria: They are spherical or elongated or rod-like cell organelle. Mitochondria were first discovered by Hofmeister in the cells of a pteridophyte, Equisetum. They were named ‘Mitochondrion’ by Benda.
Mitochondria is a double membranous organelle, the outer membrane is smooth while the inner one is folded into a number of cristae. The space between the two membranes is called the outer chamber while the space surrounded by the inner membrane is an inner chamber that is filled with homogenous fluid. The inner membrane has a large number of F, particles or exosomes.
These are the sites for oxidative phosphorylation. Each Oxysome has a head, stalk, base; The number of elementary particles in a mitochondrion maybe 104-105. The head of F, particle contain ATP synthetase enzyme hence they are said to be ATP particles.
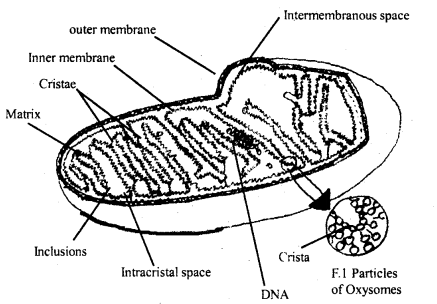
Mitochondria from animal cell
Functions of mitochondria:
- A powerhouse of the cell and store energy as ATP.
- Several respiratory enzymes are found in mitochondria.
- DNA is also contained in mitochondria.
- They regulate the concentration of calcium ions in the cells.
Question 8.
Explain in detail the structure of a typical eukaryotic chloroplast.
Answer:
Chloroplast: Chloroplasts are relatively large organelles that are visible under the light microscope. The chloroplasts are green in color and vary in size and shape from species to species. In higher plants, chloroplasts measure 2 to 4 × 5 to 10 μ in size.
Details structure of chloroplast: They are bounded by two mem-branes about 300 Å in total thickness. Each membrane is about 40 to 60 Å thick. Both the membranes are separated by a clear space of about 25-27 Å. The inner membrane is very intricately elaborated to form a system of lamellae.
Internally the chloroplasts are divided into two parts
(a) Stroma and
(b) the membrane system.
The thylakoids are closely packed in certain areas. They appear as
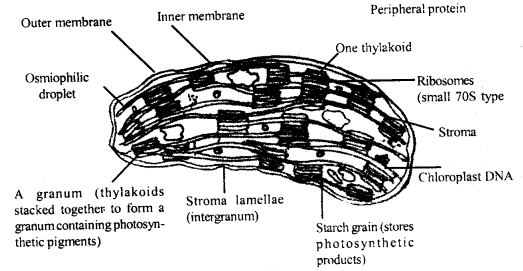
Structure of chloroplast
piles of coins placed one above the other. These structures are called grana. As many as 40 to 60 grana may be present in a single chloroplast and each granum may contain 2 to 100 coins like thylakoids. Thylakoids can be seen in a variety of configurations in different species of plants.
The arrangement can be in the form of simple parallel Sacuniiing lengthwise or maybe in a complex interconnecting network of the seek. The chloroplasts invariably have some starch granules which often accumulate near a special region known as pyrenoid in algae.
![]()