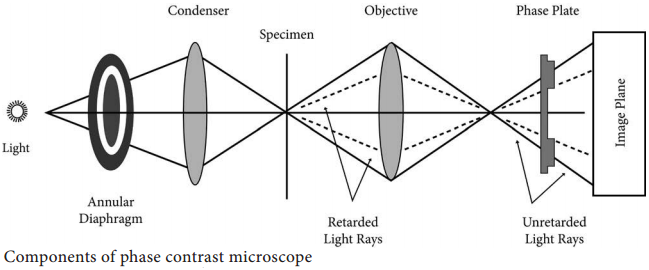
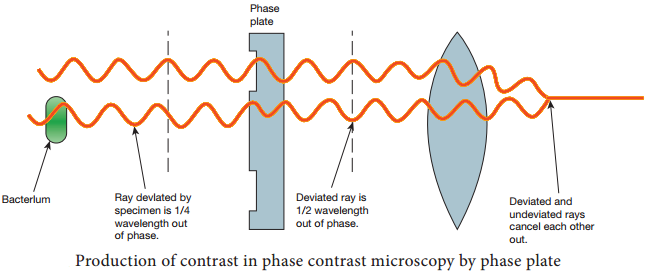
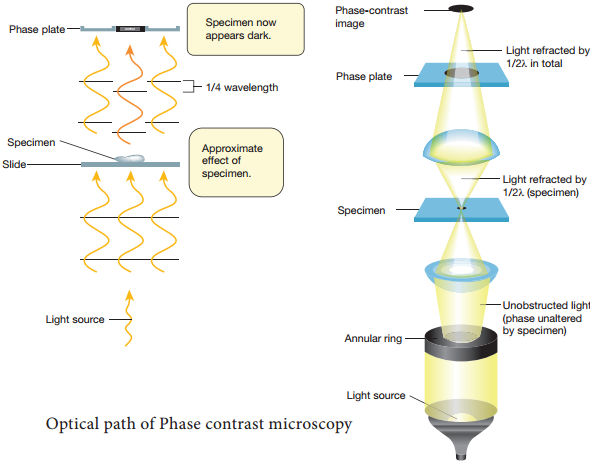
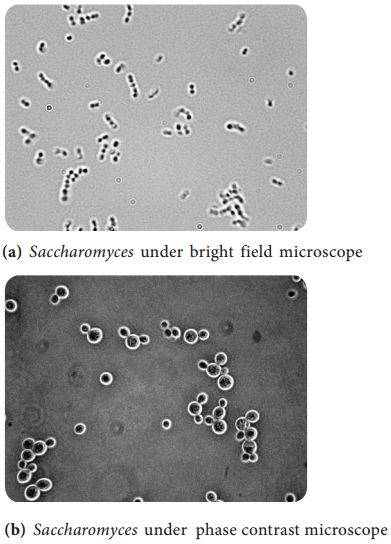
Learninsta presents the core concepts of Microbiology with high-quality research papers and topical review articles.
Fluorescence Microscope – Definition, Principle, Parts, Uses
Fluorescence microscope is a very powerful analytical tool that combines the magnifying properties of light microscope with visualization of fluorescence.
Fluorescence microscope is a type of light microscope which instead of utilizing visible light to illuminate specimens, uses a higher intensity (lower wavelength) light source that excites a fluorescent molecule called a fluorophore (also known as fluorochrome).
Fluorescence is a phenomenon that takes place when the substances (fluorophore) absorbs light at a given wavelength and emits light at a higher wavelength. Thus, fluorescence microscopy combines the magnifying properties of the light microscope with fluorescence technology.
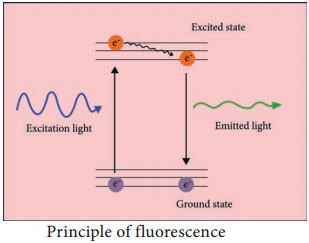
The fluorophore absorbs photons leading to electrons moving to a higher energy state (excited state). When the electrons return to the ground state by losing energy, the fluorophore emits light of a longer wavelength (Figure 2.5). Three of the most common fluorophores used are Diamidino – phenylindole (DAPI) (emits blue), Fluorescein isothiocyanate (FITC) (emits green), and Texas Red (emits red).
Principle
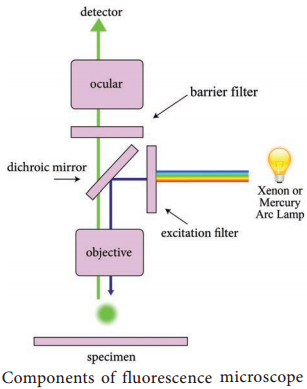
Light source such as Xenon or Mercury Arc Lamp which provides light in a wide range of wavelength, from ultraviolet to the infrared is directed through an exciter filter (selects the excitation wavelength). This light is reflected toward the sample by a special mirror called a dichroic mirror, which is designed to reflect light only at the excitation wavelength.
The reflected light passes through the objective where it is focused onto the fluorescent specimen. The emissions from the specimen are in turn, passed back up through the objective where magnification of the image occurs and through the dichroic mirror.
This light is filtered by the barrier filter, which selects for the emission wavelength and filters out contaminating light from the arc lamp or other sources that are reflected off from the microscope components. Finally, the filtered fluorescent emission is sent to a detector where the image can be digitized.
Components of Fluorescence
Microscope:
The main components of the fluorescent microscope resemble the traditional light microscope. However, the two main difference are the type of light source used and the use of the specialized filter
elements (Figure).
Light source:-
Fluorescence microscopy requires a very powerful light source such as a Xenon or Mercury Arc Lamp. The light emitted from the Mercury Arc Lamp 10 – 100 times brighter than most incandescent lamps and provides light in a wide range of wavelengths from ultra-violet to the infrared. Lasers or high-power LEDs were mostly used for complex fluorescence microscopy techniques.
Filter elements:-
A typical fluorescence microscope consists of three filters: excitation, emission and the dichroic beam splitter.
Excitation filters:
It is placed within the illumination path of a fluorescence microscope. Its purpose is to filter out all
wavelength of the light source, except for the excitation range of the fluorophore in the sample or specimen of interest.
Emission filters:-
The emission filter is placed within the imaging path of a fluorescence microscope. Its purpose is to filter out the entire excitation range and to transmit the emission range of the fluorophore in the specimen.
Dichroic filter or beam splitter:-
The dichroic filter or beam splitter is placed in between the excitation filter and emission filter, at 45° angle. Its purpose is to reflect the excitation wavelength towards the fluorophore in the specimen, and to transmit the emission wavelength towards the detector.
Working Mechanism
The specimen to be observed are stained or labeled with a fluorescent dye and then illuminated with high intensity ultra violet light from mercury arc lamp. The light passes through the exciter filter that allows only blue light to pass through. Then the blue light reaches dichroic mirror and reflected downward to the specimen.
The specimen labeled with fluorescent dye absorbs blue light (shorter wavelength) and emits green light. The emitted green light goes upward and passes through dichroic mirror, reflects back blue light and allows only green light to pass the objective lens then it reaches barrier filter which allows only green light. The filtered fluorescent emission is sent to a detector where the image can be digitized Figure.
Application
- Fluorescence microscope has become one of the most powerful techniques in biomedical research and clinical pathology.
- Fluorescence microscope allows the use of multicolour staining, labeling of structures within cells, and the measurement of the physiological state of a cell.
- Fluorescence microscope helps in observing texture and structure of coal.
- To study porosity in ceramics, using a fluorescent dye.
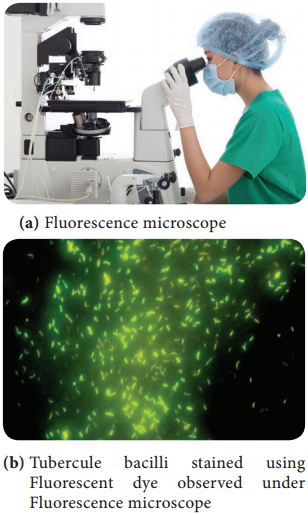
- To identify the Mycobacterium tuberculosis.