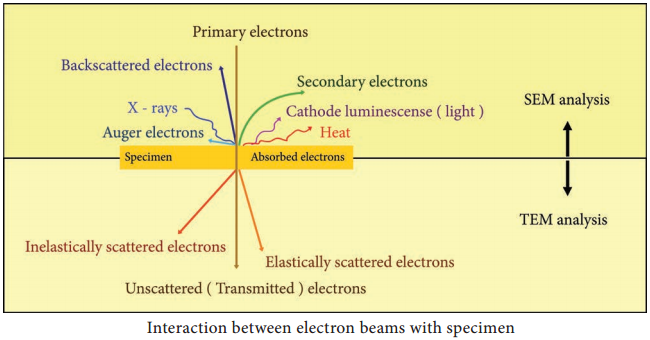
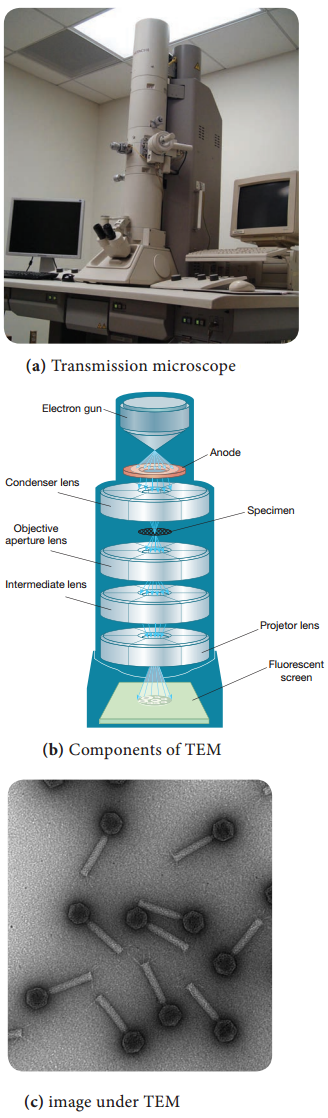
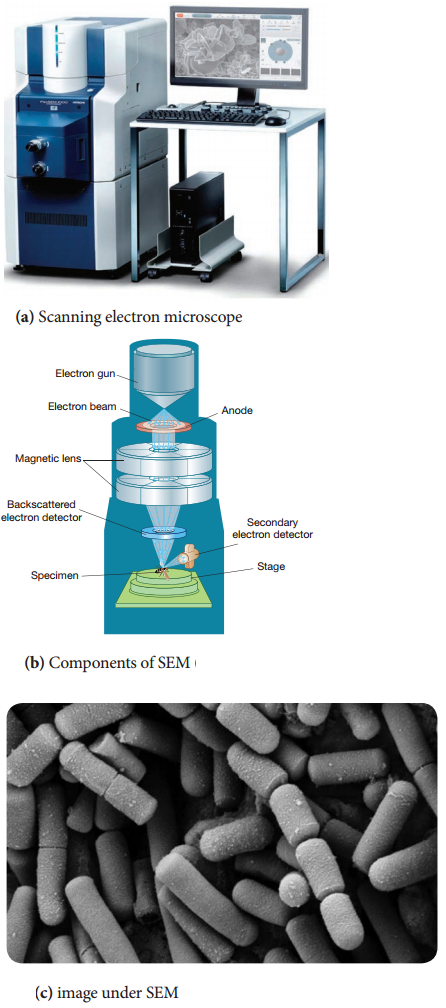
Learninsta presents the core concepts of Microbiology with high-quality research papers and topical review articles.
Major Groups of Antimicrobial Chemical Agents
A large number of chemical agents are in common use. Some of the more common groups are listed below.
1. Phenol and Phenolics
Phenol was the first widely used chemical antiseptic and disinfectant. In 1867, Joseph Lister employed carbolic spray to reduce the risk of infection in surgical theatres. Phenol derivatives called phenolics contain altered molecules of phenol useful as antiseptics and disinfectants. The phenolics damage cell membranes and inactivate enzymes of microorganisms, while denaturing the proteins.
Phenolics includes cresols, such as Lysol, as well as several bisphenols, such as hexachlorophene. Today phenol and phenolics such as cresol, xylenol, and orthophenyl phenol are used as disinfectants in laboratories and hospitals.
The commercial disinfectant Lysol is made of mixture of phenolics. Phenolics are tuberculocidal, effective in the presence of organic material, and remain active on surfaces long after application. However, they have a disagreeable odour and can cause skin irritation.
Hexachlorophene is one of the most popular antiseptics because it persists on the skin once applied and reduces skin bacteria for a long period. It is mainly used in soaps and creams. It is an ingredient of various dermatological preparation used for skin disorders.
2. Alcohols
Alcohols are among the most widely used disinfectant and antiseptic. They are bactericidal and fungicidal but not sporicidal. Alcohols can destroy the lipid component of enveloped viruses. The two most popular alcoholic germicides are ethanol and isopropanol. They act by denaturing proteins and dissolving membrane lipids. The recommended optimum concentration of ethanol is 70%, but concentration between 60% and 95% are employed to kill germs as well. Thermometers and small instruments are disinfected by immersing in alcohol for 10 to 20 minutes.
3. Halogens
Halogen compounds are broad spectrum compounds that are considered low toxicity, low cost and easy to use. Among the halogens, iodine and chlorine are important antimicrobial agents. Small quantities of drinking water can be disinfected with halazone tablets.
a. Iodine:-
Iodine compound are broad spectrum and considered effective for a variety of bacteria, mycobacteria, fungi and viruses. The alcoholic tincture of iodine is highly active against gram positive organisms and so is used as a skin antiseptic. It stains the skin. Iodine combines with microbial protein and inhibits their function.
b. Chloride:-
Chloride is also used as a gas to maintain a low microbial count in drinking water. Chlorine together with ammonia called chloramines are used to sanitize glasswall and eating utensils. Sodium hypochlorite (NaOCl) is one of the most widely used chlorine containing disinfectants. Low concentrations (2-500ppm) are active against vegetative bacteria, fungi and most viruses.
Rapid sporicidal action can be obtained around 2500ppm, however this concentration is very corrosive so should be limited in its use. High concentrations are also irritating to the mucous membranes, eyes and skin. Chlorine compounds are rapidly inactivated by light and some metals so fresh solutions should always be used. Hypochlorites should never be mixed with acids or ammonia as this will result in the release of toxic chlorine gas.
c. Iodophores:-
The combinations of iodine and organic molecules are called Iodophores. They include wescodine, betadine and previdone. These iodophore contains surface active agents. They cause less irritation to the skin than free Iodine and do not stain. They are used for cleaning wounds and as a general purpose laboratory disinfectant for discarded jars.
4. Heavy Metals
For many years the ions of heavy metals such as mercury, silver, arsenic, zinc, and copper were used as germicides. More recently these have been superseded by other less toxic and more effective germicides. Many heavy metals are more bacteriostatic than bactericidal. There are a few exceptions. 1% solution of Silver nitrate is often applied to the eyes of infants to prevent ophthalmic gonorrhea. Silver sulfadiazine is used on burns. Copper sulfate is an effective algicide used in lake and swimming pools to retard the growth of algae.
Heavy metals combine with sulfhydryl (SH) groups of proteins and inactivate them. High concentration of metallic salts, particularly those of mercury, silver and copper coagulate cellular proteins that results in damage or death of the microbial cell. The most toxic heavy metals are the mercury, silver, and copper.
5. Quaternary Ammonium Compounds (Quats)
The most widely used surface active agents are the cationic detergents, especially the quaternary ammonium compounds (quats).
Quaternary Ammonium compounds are strongly bactericidal against Gram positive bacteria and less active against gram negative bacteria. These include agents such as cetrimide, bromide and benzalkonium chloride. Their antibacterial activity is antagonized by soaps and certain organisms like Pseudomonas.
They are useful for washing cutlery in catering industry and for cleaning wounds in hospitals. Savlon, a popular antiseptic, is a mixture of cetrimide and chlorohexidine and is active against Gram negative bacteria. They are used as skin disinfectants and as a preservative of ophthalmic solution.
The combined properties of germicidal activity and low toxicity, high solubility in water, stability in solution and non-corrosiveness have resulted in many applications of quaterneries as disinfectants and sanitizing agents.
Quats are also fungicidal, amoebicidal, and virucidal against enveloped viruses. They do not kill endospores or mycobacteria.
6. Aldehydes
Aldehydes are highly effective, broad spectrum disinfectant. The most which typically achieve its anitimicrobial action by denaturing proteins and disrupting nucleic acids. Commonly used aldehydes are formaldehyde and glutaraldehyde. Formaldehyde is usually dissolved in water or alcohol before use. Formaldehyde is used as a surface disinfectat and a fumigant and has been used to decontaminate in animate objects.
A concentration of 2% glutaraldehyde is an effective disinfectant. It is less irritating than formaldehyde and is used to disinfect hospital and laboratory equipments. Glutaraldehyde usually disinfects objects about 10 minutes but may require as long as 12 hours to destroy all spores.
These are highly reactive molecules that combine with nucleic acids and proteins and inactivate them. They disrupt the function of cell organelles and kill the cells probably by cross linking and alkylating the molecules. These are sporicidal and can be used as chemical sterilants.
7. Gaseous Sterilization
Gaseous disinfectants (alkylating agents) are used for the sterilization or disinfection of hospital equipment that is bulky or heat labile. The most widely used gases are ethylene oxide, formaldehyde and β Propiolactone.
Ethylene oxide (EtO):-
Ethylene oxide has a boiling point of 10.8°C. It is highly inflammable and explosive in pure form, but is safe to handle when mixed with Carbon dioxide. It is powerful in the killing of all bacteria, including tubercule bacilli and spores. It is an effective sterilizing agent because it rapidly penetrates packing materials, even
plastic wrappers. To be potent, however, the humidity and temperature must be carefully controlled within narrow limits.
It is highly toxic on contact with the skin or mucous membrane. Materials that have been sterilized with ethylene oxide must be set aside in detoxification chambers for a few days to allow the gases to dissipate. It is frequently used to sterilize heart lung machines and plastic items like catheters.
Formaldehyde:-
It is highly bactericidal. Formaldehyde is used as 40% formalin with humidity at around 50%. It causes irritation. It is used occasionally to fumigate rooms and disinfect respirators.
Betapropiolactone (BPL):-
This is occasionally employed as a sterilizing gas in the liquid form. It has been used to sterilize vaccines, tissue grafts, surgical instrument and enzyme as a sterilants of blood plasma, water, milk and as a vapour – phase disinfectant in enclosed spaces, short-term inhalation exposure to betapropiolactone causes
severe irritation of the eyes, nose, throat and respiratory tract in humans.
BPL decomposes to an inactive form after several hours and is therefore not difficult to eliminate. It destroys microorganisms more readily than ethylene oxide but does not penetrate materials well and may be carcinogenic. For these reasons, BPL has not been used as extensively as EtO.