Learninsta presents the core concepts of Microbiology with high-quality research papers and topical review articles.
Evaluation of Antimicrobial Chemical Agents Antibiotics
Testing of antimicrobial agents is a complex process regulated by two different federal agencies.
The U.S. Environmental Protection Agency regulates disinfectants, where as agents used on humans and animals are under the control of the Food and Drug Administration.
Testing of antimicrobial agents often begins with an initial screening test to see if they are effective and at what concentrations.
Laboratory techniques for the evaluation of antimicrobial chemical agents are conducted by one of the following three general procedures. In each procedure, the chemical agent is tested against a specific microorganisms referred to as the test organism.
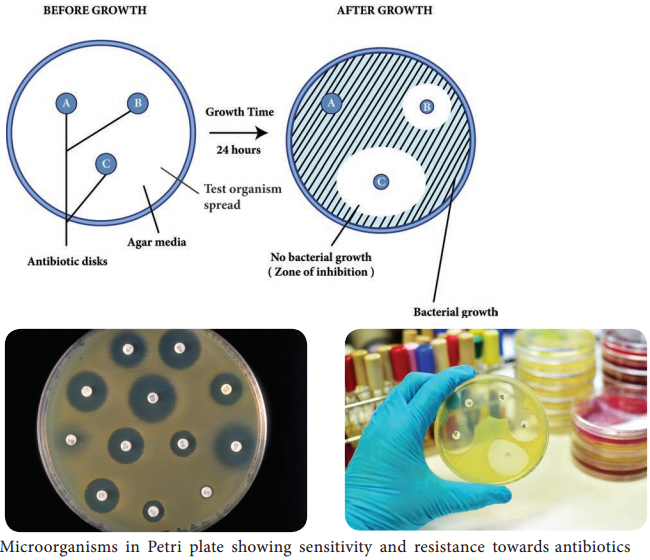
Agar Plate Method
A plate of agar medium is inoculated with the test organism and the chemical agent is placed on the surface of the medium. The chemical solution is first impregnated in absorbent papers or confined by a hollow cylinder placed on the agar surface. Following incubation, the plate is observed for a zone of inhibition around the chemical agent. This is particularly suitable for semisolid preparations.
Tube Dilution Methods
Appropriately diluted water soluble liquid substances are dispensed into sterile test tubes and are inoculated with a measured amount of the test organism. At specified intervals, a transfer is made from this tube into tubes of sterile media that are then incubated and observed for the appearance of growth.
It is necessary in this type of procedure to ascertain whether the inhibitory action is bactericidal and not bacteriostatic. This approach can also be used to determine the number of organisms killed per unit time by performing a plate count on samples taken at appropriate intervals.
Phenol Coefficient Test
Phenol coefficient is a measure of the bactericidal activity of a chemical compound in relation to phenol. Phenol coefficient is calculated by dividing the concentration of test disinfectant at which it kills the organism in 10 minutes and not in 5 minutes under the same conditions. This method is used for evaluating the efficiency of watermiscible disinfectants.
Series of 10 test tubes with 2ml of distilled water is taken (Figure 3.1). Phenol is added to first test tube and dilution is made by transferring 1ml to next tube up to 5 dilutions. Similarly commercial disinfectant is also diluted. Pure culture of test organisms, such as Staphylococcus aureus or Salmonella typhi, is added to test tubes.
Subcultures from these tubes incubated at 37°C for 48 hours are examined for the presence or absence of growth at intervals of 5, 10 and 15 minutes. The highest dilution that kills the bacteria after 10 minutes, but not after 5 minutes is used to calculate the phenol coefficient (Table 3.3),
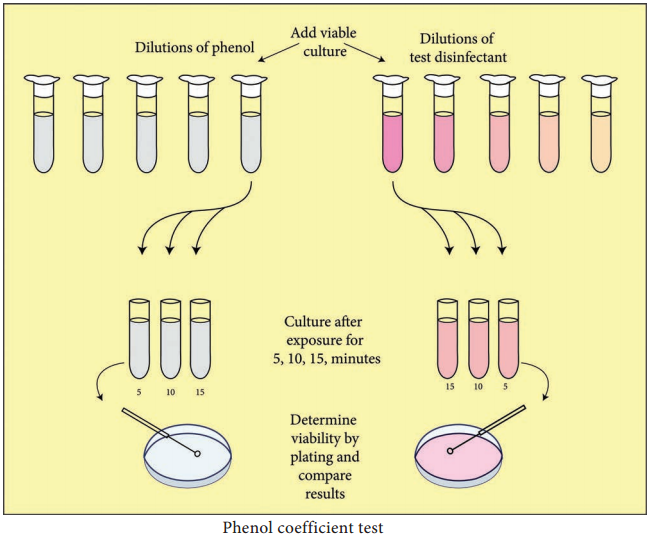
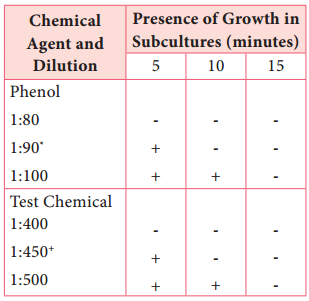
Illustration of phenol coefficient determination
Phenol dilution of 1:90 showed growth at 5 minutes but no growth at 10 minutes Test Chemical dilution of 1:450 showed growth at 5 minutes but no growth at 10 minutes phenol coefficient of test chemical as 450/90=5.
Antibiotics
The term ‘antibiotic’ was derived from ‘antibiosis’ which refers to the suppression of microorganisms due to secretion of toxic or inhibitory compounds by other microorganisms. Although antibiosis has been observed by many scientific workers fairly frequently towards the end of the nineteenth century, it was not until the discovery and development of Penicillin that a truly wide ranging search for antibiotics was initiated.
Historical Development
The first chemotherapeutic agent, discovered by Paul Ehrlich, was Salvarsan, used to treat syphilis. Alexander Fleming discovered the first antibiotic, penicillin, in 1929; its first clinical trails were done in 1940. Antibiotics are produced by species of Streptomyces, Bacillus, Penicillium and Cephalosporium.
Drugs such as the sulfonamides are sometimes called antibiotics although they are synthetic chemotherapeutic agents which are not synthesized using microbes.
Classification of Antibiotics
The antibiotics are usually classified on the basis of:-
• Target group of microorganisms
• Antimicrobial spectrum and
• Mode of action
Classification based on target group of microorganisms:-
Based on the target group, the antibiotics can be classified as antibacterial, antifungal and antiviral.
Classification based on Antimicrobial spectrum:-
Antimicrobial spectrum or antibiotic spectrum refers to the range of effectiveness of antibiotics on different kind of microorganisms, i.e. the range of different kind of microorganisms that can be inhibited, killed, or lysed by a particular type of antibiotic.
The susceptibility of microorganisms to individual antibiotic varies significantly and on account of this, the antibiotics can be classified in two groups as,
Broad – spectrum antibiotics:-
These attack different kinds of microbial pathogens and therefore find wider medical use. Antibacterial antibiotics of broad – spectrum are effective against both Gram positive and Gram negative bacteria.
They also attack pathogens belonging to Mycobacteria, Rickettsia, and Chlamydia. Similarly, broad – spectrum antifungal antibiotics attack different type of fungal pathogens.
Narrow – spectrum antibiotics:-
Narrow – spectrum antibiotics are categorized as those that are effective only against a limited variety of microbial pathogens. These antibiotics are quite valuable for the control of microbial pathogens that fail to respond to other antibiotics. For example, vancomycin is a narrow spectrum glycopeptide. It is an effective bactericidal agent for gram – positive penicillin resistant bacterial pathogens belonging to genera Staphylococcus, Bacillus, and Clostridium.
Mode of Action of Antibiotics
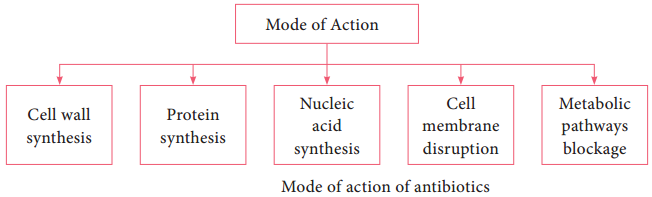
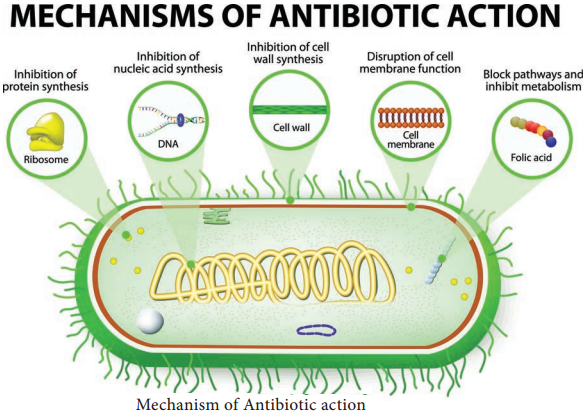
The mode of action of antibiotics varies as they damage pathogens in several ways (Flowchart 3.1). Some of the important actions of therapeutic drugs in microbial pathogens are as follows. Cell wall synthesis, Protein synthesis, Nucleic acid synthesis, Cell membrane disruption and Metabolic pathways blockage.
1. Inhibition of Cell Wall Synthesis
The most selective therapeutic antibiotics are those that interfere with the synthesis of bacterial cell walls. These drugs posses a high therapeutic index because bacterial cell walls have a unique structure which is not found in eukaryotic cells. The important cell wall attacking drugs are Penicillin, Cephalosporin, Ampicillin,
Methicillin and Vancomycin.
2. Inhibition of Protein Synthesis
Many therapeutic antibiotics discriminate between prokaryotic and eukaryotic ribosomes and inhibit protein synthesis. The therapeutic index of these drugs is fairly high, but not as favourable as that of cell wall synthesis inhibitors. Several of these drugs are medically useful and effective research tools because they block individual steps in protein synthesis. Some therapeutic drugs bind to 30S while others attach to 50S ribosomal subunits. Example Streptomycin, Chloramphenicol, Tetracyclin and Erythromycin.
3. Inhibition of Nucleic Acid Synthesis
Some antimicrobial drugs or antibiotics inhibit nucleic acid synthesis. These are not selectively toxic as other drugs. This is due to the fact that prokaryotic and eukaryotic nucleic acid synthesis mechanisms do not vary greatly. Example Quinolones, Novobiocin, Actinomycin and Rifampin
4. Disruption of Cell Membrane
There are some antimicrobial drugs or antibiotics that act as cell membrane disorganizing agents. Polymyxins are such drugs of clinical importance. E.g. Polymyxin B and Polymyxin E (colistin)
5. Blocking Metabolic Pathways
Some therapeutic drugs act as antimetabolites and block the functioning of metabolic pathways. They competitively inhibit the key enzymes in the metabolic pathway. Example Sulfonamides, Trimethoprim, Dapsone and Isoniazid (Figure 3.2).
Trimethoprim, Daspone and Isoniazid (Figure 3.2).