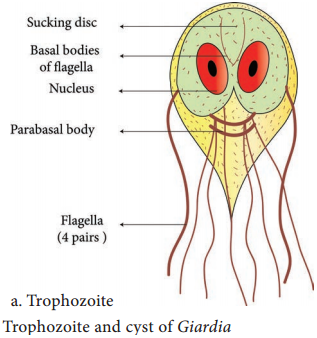
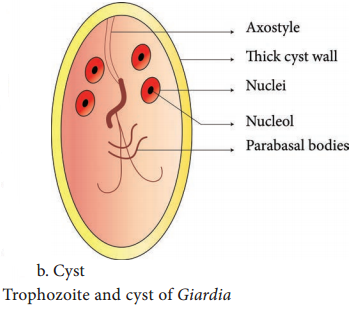
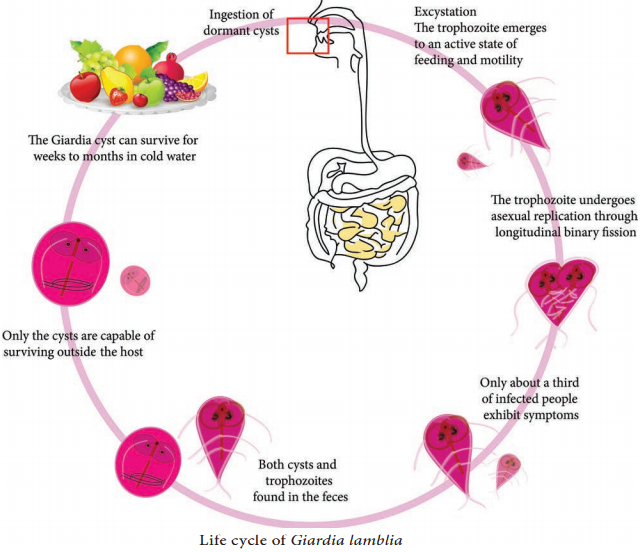
Learninsta presents the core concepts of Microbiology with high-quality research papers and topical review articles.
Plasmodium Falciparum and P. vivax (Sporozoa – Plasmodium)
Protozoan parasites characterised by the production of spore – like oocysts containing sporozoites were known as sporozoa. The parasites belonging to this group of protozoa do not possess any special organs of locomotion, such as flagella or cilia. The medically important parasite of this class that is given in the
text is malarial parasite.
Malaria
It is the disease condition with seasonal intermittent fevers, chills and shivering. The name malaria (Mal: bad, aria: air) was given in the 18th century in Italy. The specific agent of malaria was discovered in RBC’s of a patient in 1880 by Alphonse Laveran.
In 1897, Ronald Ross identified the developing stages of malarial parasites in mosquitoes in Secunderabad, India. This led to various measures for the control and possible eradication of malaria by mosquito control. Both Ross (1902) and Laveran (1907) won the Nobel Prize for their discoveries in malaria.
Causative agents of human malaria:
The organisms: Four species of Plasmodium cause malaria in humans.
- Plasmodium vivax: (Benign Tertian malaria)
- Plasmodium falciparum: (Malignant tertian malaria)
- Plasmodium malaria: (Benign Quartan malaria)
- Plasmodium ovale: (Benign tertian malaria)
The two most common species are P. vivax and P. falciparum, WHO reports (2018) that falciparum being the most pathogenic of all.
Geographical Distribution
Malarial parasites are found in all countries. In India, malaria continues to be a major public health threat.
Habitat
The malarial parasites infecting man, after passing through a developmental phase in the parenchyma cells of the liver, reside inside the red blood corpuscles and are carried by the circulating blood to all the organs.
Vectors
Human malaria is transmitted by over 60 species of female Anopheles mosquito.
Human malarial parasite – Plasmodium falciparum
Of all the human malaria parasites, P. falciparum is the most highly pathogenic and responsible for malignant tertian malaria. This is a form of disease which runs an acute course in non-immune patients and is frequently fatal if untreated.
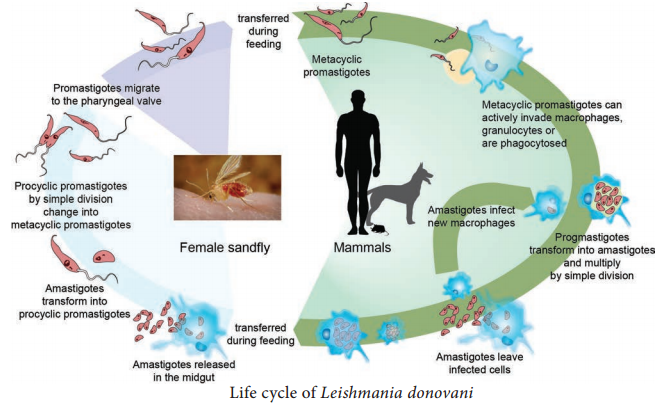
Life Cycle
The malaria parasite passes its life cycle in two different hosts and comprises of two phase as follows,
Definitive host:
Female Anopheles mosquito (a sexual phase of parasite occurs).
Intermediatehost:
Human (an asexual phase of parasite occurs). Thus, life cycle of malaria parasite show alternation of generations – asexual and sexual generation in two different hosts (Figure 8.12).
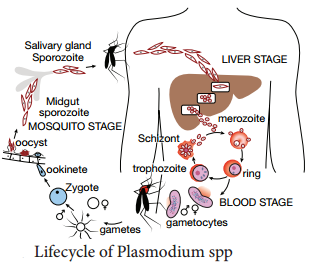
Human Cycle (Asexual Phase – Schizogony)
Human infection occurs when the sporozoites (the infective forms of the parasite are present in the salivary gland of the mosquito) are injected into blood capillaries when the mosquito feeds on blood after piercing the skin. The malarial parasite multiplies by division and the process designated as Schizogony (schizo: to split, gone: generation).
Sporozoites are minute thread-like curved organisms with tapering ends. Measuring 9-12µ in length with a central elongated nucleus while, the cytoplasm reveals no pigment as seen with a light microscope. In human, schizogony occurs in two locations. One in the red blood cells (erythrocytic schizogony) and other
in the liver cells (pre – or exoerythrocytic schizogony).
A. Pre-erythrocytic or Exoerythrocytic schigony
- Sporozoites do not directly enter the RBC’s to initiate erythrocytic schizogony, but undergo developmental phase in other human tissues.
- This cycle lasts for about 8 days in Plasmodium vivax, 6 days in P. falciparum and 9 days in P. ovale.
- This pre-erythrocytic schizogony occurs within parenchymal cells of the liver.
- The Sporozoites, which are elongated spindle – shaped bodies, become rounded inside the liver cells.
- They enlarge in size and undergo repeated nuclear division to form several daughter nuclei, each of which is surrounded by cytoplasm.
- This stage of the parasite is called the pre-erythrocytic or exoerthrocytic schizont or merozoites.
- The heptocyte is distended by the enlarging schizont and the liver cell nucleus is pushed to the periphery.
- Mature liver stage schizonts are spherical multinucleate and contain 2000-50,000 uninucleate merozoites.
- These normally rupture in 6-15 days and release thousands of merozoites into the blood stream.
- They do not return from red blood cells to liver cells.
Plasmodium vivax and P. ovale – parasites in liver tissue are called hypnozoites.
B. Erythrocyticstage
- The merozoites released by pre-erythrocytic schizonts invade the red blood cells (Parasitaemia).
- Merozoites are pear – shaped bodies, about 1.5 µm in length.
- In the erythrocyte, the merozoite loses its internal organelles and appears as rounded body having a vacuole in the center with the cytoplasm pushed to the periphery and the nucleus at one pole. These forms are called ring forms or young trophozoites.
- The parasite feeds on the hemoglobin of the erythrocyte. They incompletely metabolize hemoglobin therefore, hematin – globin pigment or haemozoin pigment is left behind.
- The malaria pigment released when the parasitized cells rupture is taken up by recticuloendothelial cells.
- The ring form develops and becomes irregular in shape and shows amoeboid motility. This is called the amoeboid form.
- When the amoeboid form reaches a certain stage of development, its nucleus starts dividing by mitosis followed by a division of cytoplasm to become mature schizonts or merozoites.
- A mature schizont contains 8-32 merozoites and haemozoin. The mature schizont bursts releasing the merozoites into the circulation.
- The merozoites invade fresh erythrocytes within which they go through the same process of development. This cycle is called erythrocytic schizogony.
- The rupture of the mature schizont releases large quantities of pyrogens. This is responsible for the febrile paroxysms characterising malaria.
- In P. falciparum, erythrocytic schizogony always takes place inside the capillaries and vascular regions of internal organs. Therefore, in these infections, schizonts and merozoites are usually not seen in the peripheral blood.
C. Gametogony
- Some of the merozoites, after a few erythrocytic cycles do not develop into trophozoites and schizonts but they undergo sexual differentiation to develop into the gametocytes.
- Development of gametocytes takes place within the internal organs and only the mature forms appear in circulation.
- The mature gametocytes in P. falciparum are crescent shaped.
- Female gametocytes are generally more numerous and larger.
- Male gametocytes and female gametocytes are called micro gametocytes and macro gametocytes respectively.
- Gametocyte appears in 10-12 days in P. falciparum.
- The gametocytes do not cause any clinical illness in the host, but are essential for transmission of the infection.
- A person who harbors the gametocytes is referred to as a carrier or reservoir.
Mosquito Cycle (Sexual Cycle – Sporogony)
- A Female Anopheles mosquito during its blood – meal from an infected person, sucks up both the sexual and asexual forms of parasite. But, only the mature sexual forms develop and the rest die.
- The gametocytes are set free in the midgut (stomach) of mosquito and undergo further development.
- The nuclear material and cytoplasm of the male gametocyte divides to produce long, actively motile, whip – like forms of 8 microgametes. This process is called exflagellation of male gametocytes.
- The Exflagellation is completed within 15-30 minutes for P. falciparum.
- The female gametocyte does not divide but maturation involves by condensation of nucleus to become the female gamete.
- Female gamete is fertilized by one of the microgametes to produce the zygote. The zygote is formed in 20-120 minutes after the blood meal. The zygote is initially is a non – motile round body, but within 18-24 hours, it gradually elongates into a vermicular motile form. This is called the ookinete.
- Ookinete penetrates the epithelial lining of stomach wall. Their anterior end comes in close contact to the cell membrane by secretion of some proteolytic substances which causes lysis of cell membrane. Later, the ookinete come to lie just beneath the basement membrane.
- It becomes rounded into a sphere with an elastic membrane. This stage is called the oocyst. The oocyst increase in size and undergo numerous nuclear multiplication which develops a large number of sickle shaped bodies known as sporozoites.
- Number of oocysts in the stomach wall varies from a few to over a hundred.
- Around the 10th day of infection the oocyst ruptures, releasing sporozoites in the body cavity of the mosquitos.
- The sporozoites are distributed through the circulating fluid into various organs and tissues of the mosquito except the ovaries.
- The sporozoites have a special affinity towards the salivary glands. The mosquito at this stage is capable of transmitting infection to man.
Pathogenesis
In malaria, typical pathological changes are seen primarily in the spleen, liver, bone marrow, lungs, kidney and brain.
Liver:
The liver is enlarged. The organ becomes more firm and pigmented. Pigments are found in parenchymal cells.
Spleen:
The spleen is markedly enlarged. If the infection lasts over a long period, the spleen is usually grayish, dark brown or even black and is commonly known as ‘ague cake’. Bone marrow, Lungs, Kidneys and Brain are enlarged and pigmented. They are filled with parasitized erythrocytes.
Anemia is caused by destruction of large number of red cells by complement mediated and autoimmune hemolysis. It is also due to the increased clearance of both parasites and parasitized RBCs by the spleen.
Clinical Manifestations
The incubation period is generally 9-14 days but, it can be as short as 7 days. The most malignant form of malaria is caused by P. falciparum hence, variable clinical syndromes are associated with falciparum malaria. That include,
1. Prodromal (initial indication of the onset of disease) period:
Non – specific symptoms such as malaise (condition of general weakness or discomfort), myalgia
(severe muscle pain) headache and fatigue (feeling of tiredness) are usually seen during the prodromal period.
2. Malarial paroxysm (sudden onset of disease):
It is the classical manifestation of acute malaria. It is characterised by fever, chill and rigor (sudden feelings of cold with shivering).The fever is caused by rupture of red blood cells that contain malarial parasites. The fever occurs every 48 hours in falciparum malaria.
3. Anemia (A condition in which the blood does not have enough healthy Red Blood cells) and
4. Hepatosplenomegaly (simultaneously enlargement of both the liver and the spleen)
The symptoms are non – specific with headache, pains in back and limbs, anorexia, nausea and a feeling of chill rather than a distinct cold phase. Hyponatremia (A condition that occurs when the level of Sodium in the blood is too low) occur in both uncomplicated and severe malaria.
Complications of Severe
Falciparum Malaria
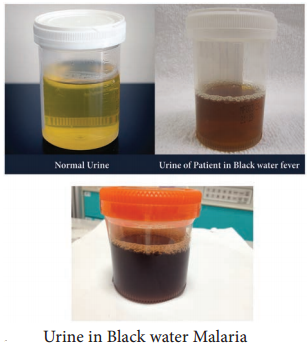
1. Black water fever
The syndrome is the manifestation of repeated infections of falciparum malaria, which were inadequately treated with quinine. The condition is associated with haemoglobinaemia (excess of hemoglobin in the blood plasma) and haemoglobinuria (excretion of free haemoglobin in the urine).
The syndrome is known as black water fever due to the dark red to brown – black appearance of the urine in this condition (Figure 8.13). It is dark due to presence of free haemoglobin as methaemoglobin or oxyhaemoglobin in it. Kidney failure is the immediate cause of death.
2. Cerebral malaria
Cerebral malaria is the most common presentation of severe malaria in adult. Cerebral malaria may be sudden in onset. Clinically, the condition manifests with fever for 4-5 days, slowly lapsing into coma, with or without convulsions.
It is marked by a severe headache, high fever even above 180°F, and changes in mental
status. Death may occur within few hours. Algid malaria and septicemic malaria are also other serious complication of falciparum malaria.
3. Pernicious malaria
The term pernicious malaria is referred to as a series of phenomena that occur during the course of an in treated P. falciparum infection within 1 to 3 days.
Anaemia:
An individual suffering from an attack of malaria, after a few paroxysms becomes temporarily anaemic. The reduction in red blood cells is greater in P. falciparum infection than in infection with P. vivax and P. malariae. This is because P. falciparum invades young and mature erythrocytes and the infection rate
of red blood cells is also greater.
Recrudescence
In P. falciparum and P. malariae infections after the primary attack, sometimes there is a period of latency, during which there is no clinical illness. But some parasites persist in some erythrocytes and gradually increase in numbers.
Fresh malarial attacks then develop. It appears after a period of latency usually within weeks after the primary attacks. Persistence of the erythrocytic cycle of the parasites are called recrudescences. In P. falciparum infections, recrudescences are seen for 1-2 years, while in P. malariae infection, they may last for long periods, even upto 50 years.
Plasmodium vivax
P. vivax shows a similar life cycle in humans and mosquitoes like that of P. falciparum. Except in P. vivax, a latent tissue stage, the hypnozoites present in the liver parenchyma. Relapse in vivax malaria is caused by these hypnozoites. Hypnozoites are the dormant stages of the parasites.
These are single – nucleated parasites measuring 4µm-6µm in diameter. These become active and develop into tissue schizonts after a short period of dormancy. This relapse may occur at intervals up to 3 years or more after the first attack. P. vivax merozoites invade only young erythrocytes and the reticulocytes.
Clinical Manifestations
P. vivax is the most wide spread species causing malaria in man. However, unlike falciparum malaria, vivax malaria, is less severe and death from the condition relatively is less common. Table 8.2 describes the comparison of course of infection in Falciparum malaria with Vivax malaria
Stage | P.falciparum | P.vivax |
| Pre-erythrocytic schizogony | Stage lasts for 6 days. Each Schizont produces about 40,000 merozoites approximately. | Lasts for 8 days. Each Schizont produces about 12,000 approximately |
| Erythrocytic schizogony | Each cycle lasts for 36–48
hours. First temperature peak occurs by 12th day of infection. Primary attack last for 10-14 days | Each cycle lasts for 48 hours. First fever peak occur by 16th day of infection. Primary attack lasts for 3-4 weeks. |
| Gemotogony | Gametocytes in peripheral blood may be seen on 21st day of infection | Gametocytes in peripheral
blood may be seen on 16th day of infection. |
| Exo – erythrocytic schizogony | Absent. Relapses do not occur | Present. Can continue for up to 3 years. Relapses often occur. |
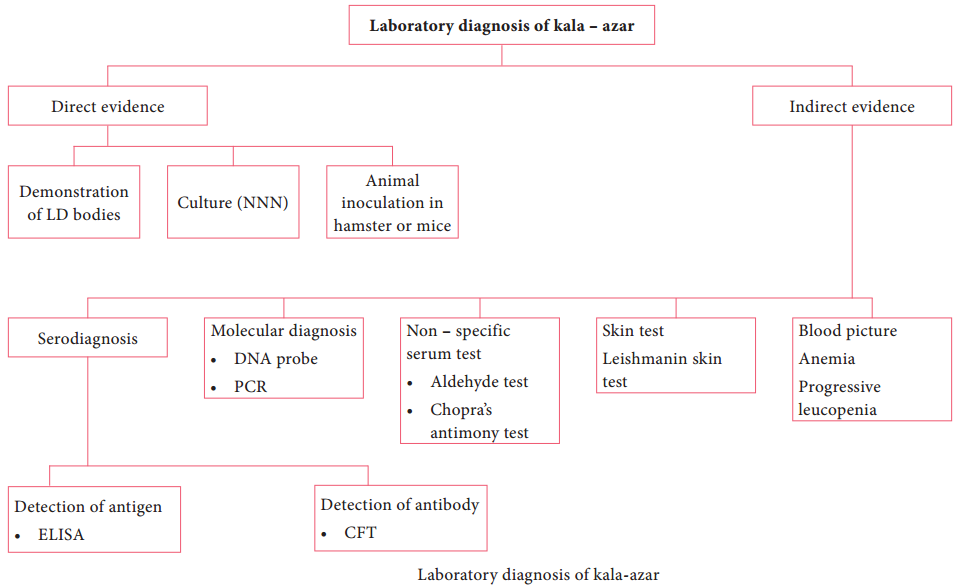
Laboratory Diagnosis
Diagnosis of malaria includes:
- Parasitic diagnosis
- Serodiagnosis, and
- Molecular diagnosis
Parasitic diagnosis – Demonstration of parasite by microscopy
Specimen:
Blood
Conventional light microscopy of stained blood smear is the gold standard for confirmation of malaria.
Two types of smears are prepared from the peripheral blood. They are thin and thick smears (Figure 8.14). Ring forms and gametocytes are most commonly seen in the peripheral blood smear. They are thin and thick smears (Figure 8.14). Ring forms and gametocytes are most commonly seen in the peripheral blood smear.
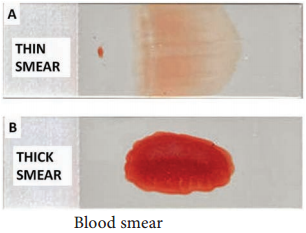
Thin smear They are prepared from capillary blood of fingertip and spread over a good quality slide by a second slide (spreader slide) held at an angle of 30°-45° from the horizontal such that a tail is formed. Thin smears thus prepared are air dried, fixed in alcohol and stained by one of the Romanowsky stains such as Leishman, Giemsa or JSB (Jaswant singh and Bhattacharjee) stain.
Thin smears are used for:
- Detecting parasites, and
- For determining the species of the infecting parasite.
Thick smear
They are prepared usually with 3 drops of blood spread over a small area of about 10mm. The thick film is dried. This smears consist of a thick layer of dehemoglobinized (lysed) red blood cells. It is not fixed in methanol. Thick film is stained similar to thin film. Thick smears have the advantage of concentrating the parasites and therefore increase the sensitivity of diagnosis. Thick smears are used for:
- Defecting parasites,
- Quantitating parasitaemia, and
- Demonstrating malarial pigments.
Fluoroscence microscopy
The method is mainly used for mass screening in field laboratory. Fluorescent dyes like acridine orange is used to stain the blood smears. It stains DNA as fluorescent green and cytoplasmic RNA as red.
QBC (Quantitative Buffy coat smear)
This is a sensitive method for detection of malaria parasites. In this method, blood is collected in a capillary tube coated with fluorescent dye and is subjected to centrifugation. After centrifugation, the Buffy coat in the centrifuged capillary tubes is examined under a fluorescent microscope. Acridine orange – stained
malaria parasites appear brilliant green.
Serodiagnosis
It is not helpful in clinical diagnosis. It is used mainly for epidemiological survey and to identify the infected donors in transfusion malaria. The test used are indirect haemagglutination (IHA), Indirect fluorescent antibody (IFA) and Enzyme – linked immunosorbent assay (ELISA) for the detection of serum antibodies.
Rapid Antigen detection tests kits are available commercially like the dipstick, card and cassette bearing monoclonal antibody. These tests are based on the detection of antigens using immune chromatographic methods. These tests can detect plasmodium in 15 minutes.
Molecular diagnosis
DNA probe and PCR are highly sensitive methods for the diagnosis of malaria. It is more sensitive than that of thick blood smear. It is highly species specific. Other tests includes the measurement of hemoglobin, total WBC and platelet count in severe falciparum malaria, urine can be tested for free hemoglobin, if black water fever is suspected. Blood urea and serum creatinine has to be monitored for renal failure.
Treatment
The most commonly used drugs are Chloroquine, Quinine, Pyrimethamine and Doxycycline.
Prevention and Control
The preventive measures to control malaria mainly depend on treatment of infected individuals and reducing the transmission of malaria. The control measures include the use of insecticides such as DDT (Di chlorodiphenyl tri chloromethane) or Malathion for controlling the populations of adult mosquitoes.
Proper use of mosquito nets, wearing protective clothings and use of mosquito repellants can prevent the mosquito bite.
Introduction to Helminths
General characteristics of Helminthic parasite:
- Helminths are multicellular worms. They are bilaterally symmetrical animals having 3 germ layers and belong to the kingdom Metazoa.
- They are invertebrates characterised by elongated, flat or round bodies.
- Helminths develop through egg, larval and adult stages. Flowchart 8.1 describes the classification of helminthes.