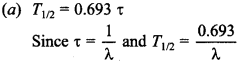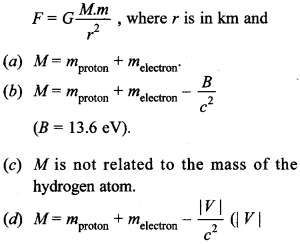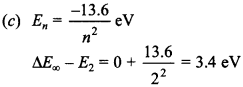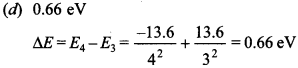Free PDF Download of CBSE Biology Multiple Choice Questions for Class 12 with Answers Chapter 12 Biotechnology and its Applications. Biology MCQs for Class 12 Chapter Wise with Answers PDF Download was Prepared Based on Latest Exam Pattern. Students can solve NCERT Class 12 Biology Biotechnology and its Applications MCQs Pdf with Answers to know their preparation level.
Biotechnology and its Applications Class 12 Biology MCQs Pdf
1. The genes crylAb and cryllAb produce toxins against _______ and _______, respectively.
(a) cotton bollworms, com borer
(b) nematode, cotton bollworm
(c) com borer, cotton bollworm
(d) com borer, nematodes
Answer
Answer: c
2. Which among the following is based on antigen-antibody interaction?
(a) PCR
(b) Electrophoresis
(c) ELISA
(d) All of these.
Answer
Answer: c
3. Which among the following is not allowed to take place in the case of RNA interference employed in making tobacco plants resistant to the nematode, Meloidegyne incognitia?
(a) Transcription of mRNA
(b) Translation of mRNA
(c) Replication of DNA
(d) Maturation of hn RNA.
Answer
Answer: b
4. Night blindness can be prevented by use of
(a) golden rice
(b) transgenic tomato
(c) transgenic maize
(d) Bt brinjal.
Answer
Answer: a
5. The Ti plasmid used for producing transgenic plants is found in
(a) Azotobacter
(b) Rhizobium
(c) Azospirillum
(d) Agrobacterium
Answer
Answer: d
6. a-1 antitrypsin is: [NCERT Exemplar]
(a) an antacid
(b) an enzyme
(c) used to treat arthritis
(d) used to treat emphysema.
Answer
Answer: d
7. The trigger for activation of toxin of Bacillus thuringiensis is [NCERT Exemplar]
(a) acidic pH of stomach.
(b) high temperature.
(c) alkaline pH of gut
(d) mechanical action in the insect gut.
Answer
Answer: c
8. In RNAi, genes are silenced using [NCERT Exemplar]
(a) ss DNA
(b) ds DNA
(c) ds RNA
(d) ss RNA
Answer
Answer: c
9. C-peptide of human insulin is [NCERT Exemplar]
(a) a part of mature insulin molecule
(b) responsible for formation of disulphide bridges
(c) removed during maturation of pro-insulin to insulin
(d) responsible for its biological activity.
Answer
Answer: c
10. Silencing of a gene could be achieved through the use of [NCERT Exemplar]
(a) short interfering RNA (RNAi)
(b) antisense RNA
(c) by both
(d) none of the above.
Answer
Answer: c
11. GM crops help to reduce the ______ losses.
Answer/Explanation
Answer:
Explaination: Post-harvest.
12. The alkaline pH in the stomach of insect larvae triggers the activation of ______ .
Answer/Explanation
Answer:
Explaination: Bt-toxin.
13. ______ is the compound obtained from transgenic animal that is used to treat emphysema.
Answer/Explanation
Answer:
Explaination: α-1-antitrypsin.
14. In RNAi, the silencing of mRNA is carried out by using ______ .
Answer/Explanation
Answer:
Explaination: ds RNA.
15. The Bt toxin does not kill the bacterium producing it, because it is produced in an _________ state.
Answer/Explanation
Answer:
Explaination: Inactive.
16. The site of production of ADA in the body is ______ .
Answer/Explanation
Answer:
Explaination: Lymphocytes.
17. ______ of human insulin is removed during maturation process.
Answer/Explanation
Answer:
Explaination: C-peptide.
18. Using ______ vectors, nematode-specific genes are introduced into host plants.
Answer/Explanation
Answer:
Explaination: Agrobacterium.
19. The protein coded by the gene, crylAb provides resistance to ______ .
Answer/Explanation
Answer:
Explaination: Com borer.
20. An American company obtained patent rights on ______ rice through US patent and Trademark Office.
Answer/Explanation
Answer:
Explaination: Basmati.
21. Match the items in Column I with those in Column II.
| Column I | Column II |
| A. Rosie | 1. Polio vaccine safety |
| B. T. plasmid | 2. Human alpha-lactalbumin |
| C. RNAi | 3. Agrobacterium tumefaciens |
| D. ELISA | 4. Meloidegyne incognitia |
| E. Transgenic mice | 5. Antigen-antibody interaction |
Answer/Explanation
Answer:
Explaination: A – 2, B – 3, C – 4, D – 5, E – 1
22. Match the terms in Column I with those in Column II.
| Column I | Column II |
| A. Gene therapy | 1. Human insulin |
| B. Cotton bollworm | 2. Biopiracy |
| C. EliLilly | 3. Emphysema |
| D. Basmati Rice | 4. ADA deficiency |
| E. α -1 antitrypsin | 5. Lepidopteran |
Answer/Explanation
Answer:
Explaination: A – 4, B – 5, C – 1, D – 2, E – 3
23. The disorder ADA deficiency can be cured by gene therapy only. [True/False]
Answer/Explanation
Answer:
Explaination: False.
24. Transgenic mice are used to test the safety of polio vaccine. [True/False]
Answer/Explanation
Answer:
Explaination: True.
25. The recombinant therapeutics induce unwanted immunological responses. [True/False]
Answer/Explanation
Answer:
Explaination: False.
26. Bt toxins provide resistance to all types of insect pests. [True/Faise]
Answer/Explanation
Answer:
Explaination: False.
27. ELISA is based on introducing a functional gene is place by a defective gene. [True/False]
Answer/Explanation
Answer:
Explaination: False.
Directions (Q28 to Q30): Mark the odd one in each of the following groups.
28. PCR, Widal, rDNA technology, ELISA.
Answer/Explanation
Answer:
Explaination: Widal.
29. Tetanus taxoid, a-1 antitrypsin, Hepatitis B vaccine, Humulin.
Answer/Explanation
Answer:
Explaination: Tetanus taxoid.
30. Agrobacterium, RNA/, crylAc, Meloidegyne.
Answer/Explanation
Answer:
Explaination: crylAc
31. What do the letters Bt stand for, in Bt cotton plants?
Answer/Explanation
Answer:
Explaination: Bt stand for Bacillus thuringiensis.
32. What are cry genes? In which organism are they present? [AI2017]
Answer/Explanation
Answer:
Explaination:
– The genes which code for the Bt toxin proteins, are called cry genes.
– They are present in the bacterium, Bacillus thuringiensis.
33. Mention the source organism of the gene crylAc and its target pest. [Foreign 2011]
Answer/Explanation
Answer:
Explaination:
– Bacillus thuringiensis is the source organism.
– Cotton bollworm is its target pest.
34. Mention the source organism of the gene, crylAb and its target pest. [Foreign 2011]
Answer/Explanation
Answer:
Explaination:
– Bacillus thuringiensis is the source 42. organism.
– Com borer is its target pest.
35. List the type of cry genes that provide resistance to com plants and cotton plants, respectively, against lepidopterans. [Foreign 2017]
Answer/Explanation
Answer:
Explaination:
– crylAb controls com borer and crylAc and cryllAb control cotton bollworm.
36. Why are certain cotton plants called Bt- cotton plants?
Answer/Explanation
Answer:
Explaination: They are the transgenic cotton plants with genes from Bacillus thuringiensis’, they are resistant to bollworms.
37. Name the specific type of gene that is incorporated in a cotton plant to protect the plant against cotton bollworm infestation. [AI 2017]
Answer/Explanation
Answer:
Explaination: crylAc and cryllAb.
38. Bt toxins are released as inactive crystals in the bacterial body. What happens to it in the cotton bollworm body that it kills the bollworm? [AI 2017]
Answer/Explanation
Answer:
Explaination:
– It becomes activated in the alkaline pH of the gut of the insect.
– The activated toxin binds to the surface of midgut epithelial cells, create pores that cause swelling and lysis of the cells and eventually cause death of the insect.
39. What is the significance of the process of RNA interference (RNAi) in eukaryotic organisms?
Answer/Explanation
Answer:
Explaination: RNA interference is a method of cellular defense in all eukaryotic organisms.
40. Write the possible source of RNA interference (RNAi) gene. [Delhi 2013C]
Answer/Explanation
Answer:
Explaination:
(i) Infection by a virus having RNA genome.
(ii) Mobile genetic elements, called transposons that replicate via an RNA intermediate.
41. How does dsRNAgain entry into eukaryotic cell to cause RNA interference? [Delhi 2011C]
Answer/Explanation
Answer:
Explaination:
(i) Infection by viruses with RNAgenome.
(ii) Transposons, the mobile genetic elements, that can replicate via an RNA intermediate.
42. How does silencing of specific mRNA in RNA interference prevent parasitic infestation?
Answer/Explanation
Answer:
Explaination: The nematode (parasite) cannot live in the transgenic host that expresses RNA interference.
43. State the role of transposons in silencing of mRNA in eukaryotic cells. [AI 2013]
Answer/Explanation
Answer:
Explaination: Transposons are the source of complementary RNA that binds to and prevents the translation of specific mRNA.
44. How are tobacco plants benefitted when nematode-specific genes are introduced into them using certain vectors? Name the vector used.
Answer/Explanation
Answer:
Explaination:
– The tobacco plants show resistance to nematode attack; the nematode cannot survive in such plants, as they show RNA interference.
– The vector used is Agrobacterium.
45. Mention the chemical change that proinsulin undergoes, to be able to act as mature insulin. [CBSE 2018]
Answer/Explanation
Answer:
Explaination:
– The C-peptide is removed.
– The peptides A and B are joined by disulphide bridges.
46. State the role of C-peptide in human insulin. [AI 2014]
Answer/Explanation
Answer:
Explaination:
The C-peptide joins the A-peptide with B-peptide in the proinsulin; it is removed during the processing of proinsulin into insulin.
47. How are the two short polypeptide chains of insulin linked together?
Answer/Explanation
Answer:
Explaination:
They are linked together by creating disulphide bonds.
48. How is proinsulin different from the functional insulin in humans? [AI 2012C]
Answer/Explanation
Answer:
Explaination:
– Proinsulin contains an additional polypeptide chain called C-peptide.
– In the functional insulin, C-peptide is absent, as it is removed during processing.
49. A boy has been diagnosed with ADA deficiency. Suggest any one possible treatment. [Delhi 2014 C]
Or
Suggest any two possible treatments that can be given to a patient exhibiting adenosine deaminase deficiency. [AI 2015]
Answer/Explanation
Answer:
Explaination:
(i) Gene therapy.
(ii) Enzyme replacement therapy.
(iii) Bone marrow transplantation.
50. State the cause of adenosine deaminase enzyme deficiency. [AI 2015]
Answer/Explanation
Answer:
Explaination: It is caused due to deletion of the gene coding for the enzyme, adenosine deaminase.
51. Why do children cured by enzyme replacement therapy of adenosine deaminase deficiency, need periodic treatment?[AI 2015]
Answer/Explanation
Answer:
Explaination: The lymphocytes are not immortal, but have a life span; hence with the formation of new lymphocytes, every time, the enzyme has to be injected.
52. Mention an example of stem cell therapy.
Answer/Explanation
Answer:
Explaination: Bone marrow transplantation.
53. Suggest a molecular diagnostic procedure that detects HTV in a suspected AIDS patient. [Foreign 2016]
Answer/Explanation
Answer:
Explaination: Enzyme-linked immunosorbent assay (ELISA)
54. Name any two techniques that serve the purpose of early diagnosis of some bacterial/ viral human diseases. [Foreign 2011]
Or
Name a molecular diagnostic technique to detect the presence of a pathogen in its early stage of infection. [Delhi 2010]
Answer/Explanation
Answer:
Explaination:
(i) Polymerase chain reaction (PCR).
(ii) Enzyme-linked immunosorbent assay (ELISA).
55. State the principle on which ELISA works.
Answer/Explanation
Answer:
Explaination: ELISA works on the principle of antigen- antibody interaction.
56. What are transgenic animals? Give an example.
Answer/Explanation
Answer:
Explaination:
– Transgenic animals are those animals which have their DNA manipulated to possess and express one/more foreign genes.
e.g. – the transgenic cow, Rosie, possesses the gene for human alpha- lactalbumin.
57. Name the protein produced in transgenic animals that is used to treat emphysema.
Answer/Explanation
Answer:
Explaination: α-1-antitrypsin.
58. What is biopiracy? [Delhi 2017, AI 2016, Delhi 2015]
Answer/Explanation
Answer:
Explaination: Biopiracy refers to the use of bioresources and traditional knowledge related to bioresources for commercial benefit by certain organisations or multi-national companies without proper consent from the countries and compensatory payment to the people concerned.
59. Name the Indian variety of rice patented by an American company.
Answer/Explanation
Answer:
Explaination: Basmati variety.
60. Mention two objectives of setting up GEAC by our government. [AI 2016]
Or
State the purpose for which the Indian Government has set up GEAC. [Foreign 2013]
Answer/Explanation
Answer:
Explaination:
The objectives of setting up GEAC are:
(a) to have some ethical standards to evaluate the morality of human activities that might help or harm living organisms.
(b) to have a regulation, as genetic modifications of organisms may have unpredictable results when such organisms are introduced into the ecosystem.
We hope the given Biology MCQs for Class 12 with Answers Chapter 12 Biotechnology and its Applications will help you. If you have any query regarding CBSE Class 12 Biology Biotechnology and its Applications MCQs Pdf, drop a comment below and we will get back to you at the earliest.