Find free online Chemistry Topics covering a broad range of concepts from research institutes around the world.
Third Law of Thermodynamics
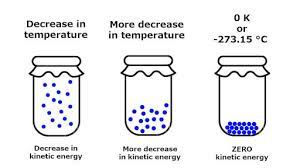
The entropy of a substance varies directly with temperature. Lower the temperature, lower is the entropy. For example, water above 100° C at one atmosphere exists as a gas and has higher entropy (higher disorder). The water molecules are free to roam about in the entire container.
When the system is cooled, the water vapour condenses to form a liquid. The water molecules in liquid phase still can move about somewhat freely. Thus the entropy of the system has decreased. On further cooling, water freezes to form ice crystal.
The water molecules in the ice crystal are highly ordered and entropy of the system is very low. If we cool the solid crystal still further, the vibration of molecules held in the crystal lattice gets slower and they have very little freedom of movement (very little disorder) and hence very small entropy.
At absolute zero {0 K (or) – 273°C}, theoretically all modes of motion stop.
Absolute zero is a temperature that an object can get arbitrarily close to but absolute zero will remain unattainable.
Thus the third law of thermodynamics states that the entropy of pure crystalline substance at absolute zero is zero. Otherwise it can be stated as it is impossible to lower the temperature of an object to absolute zero in a finite number of steps. Mathematically,
![]() = 0 for a perfectly ordered crystalline state.
= 0 for a perfectly ordered crystalline state.
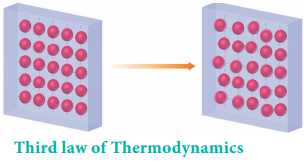
Crystals with defects (imperfection) at absolute zero, have entropy greater than zero. Absolute entropy of a substance can never be negative.
In simple terms, the third law states that the entropy of a perfect crystal of a pure substance approaches zero as the temperature approaches zero. The alignment of a perfect crystal leaves no ambiguity as to the location and orientation of each part of the crystal.
The third law of thermodynamics states that the entropy of a system approaches a constant value as the temperature approaches absolute zero.
Third law of thermodynamics statement states that the entropy of a pure crystal at absolute zero is zero. An example that states the third law of thermodynamics is vapours of water are the gaseous forms of water at high temperature. The molecules within the steam move randomly.
The third law of thermodynamics has two important consequences: it defines the sign of the entropy of any substance at temperatures above absolute zero as positive, and it provides a fixed reference point that allows us to measure the absolute entropy of any substance at any temperature.
The second law of thermodynamics states that the entropy of any isolated system always increases. The third law of thermodynamics states that the entropy of a system approaches a constant value as the temperature approaches absolute zero.
Entropy is related to the number of possible microstates, and with only one microstate available at zero kelvin the entropy is exactly zero. The third law was developed by the chemist Walther Nernst during the years 1906-1912. It is often referred to as Nernst’s theorem or Nernst’s postulate.