Find free online Chemistry Topics covering a broad range of concepts from research institutes around the world.
s-Block Elements
The elements belonging to the group 1 and 2 in the modern periodic table are called s-block elements. The elements belonging to these two groups are commonly known as alkali and alkaline earth metals respectively. In this unit, we study their properties, uses, important compounds and biological importance.
S-block comprises 14 elements namely hydrogen (H), lithium (Li), helium (He), sodium (Na), beryllium (Be), potassium (K), magnesium (Mg), rubidium (Rb), calcium (Ca), cesium (Cs), strontium (Sr), francium (Fr), barium (Ba), and radium (Ra).
s-block elements are the elements found in Group 1 and Group 2 on the periodic table. Group 1 are the alkali metals which have one valence electron. They have low ionization energies which makes them very reactive. Group 2 is the alkali earth metals which have two valence electrons, filling their s sublevel.
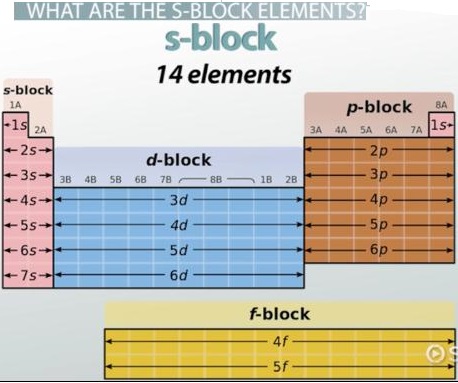
The s-block and p-block elements are so called because their valence electrons are in an s orbital or p orbital respectively. They are also called Typical Elements to distinguish them from the transition and inner transition series.
Elements in which all inner electron shells are completely filled, and the last electron enters the s-orbital of the outermost shell, are called s-block elements. Thus, for s-block elements, the differentiating electron enters the ns-orbital.
The s-block in the periodic table of elements occupies the alkali metals and alkaline earth metals, also known as groups 1 and 2. Helium is also part of the s block. The principal quantum number “n” fills the s orbital. There is a maximum of two electrons that can occupy the s orbital.
Physical properties of S-Block Elements
- Electronic Configuration.
- Large Atomic Radii.
- Large Ionic Radii.
- Low Ionization Enthalpy.
- Hydration Enthalpy.
- Unipositive ions.
- Metallic Character.
- Melting and Boiling Points.
The s block has two columns corresponding to one of the s orbitals holding a maximum of two electrons. The p block has six columns corresponding to the three p orbitals with two electrons each. The lettered group number of a main-group element is equal to the number of valence electrons for that element.
The s-block is on the left side of the conventional periodic table and is composed of elements from the first two columns plus one element in the rightmost column, the nonmetals hydrogen and helium and the alkali metals (in group 1) and alkaline earth metals (group 2). Their general valence configuration is ns1-2.
S Block Elements are a family of elements with similar characteristcs. S Block Elements include Alkali Metals. They include Lithium, Sodium, Potassium, Rubidium, Cesium, and Francium. They display many of the physical properties similar to metals.
As the s-orbital can accommodate only two electrons, two groups (1 & 2) belong to the s-block of the Periodic Table. Group 1 of the Periodic Table consists of the elements: lithium, sodium, potassium, rubidium, caesium and francium. They are collectively known as the alkali metals.
There can be two electrons in one orbital maximum. The s sublevel has just one orbital, so can contain 2 electrons max. The p sublevel has 3 orbitals, so can contain 6 electrons max. The d sublevel has 5 orbitals, so can contain 10 electrons max.
Recall that the four different sublevels each consist of a different number of orbitals. The s sublevel has one orbital, the p sublevel has three orbitals, the d sublevel has five orbitals, and the f sublevel has seven orbitals. In the first period, only the 1s sublevel is being filled.