Find free online Chemistry Topics covering a broad range of concepts from research institutes around the world.
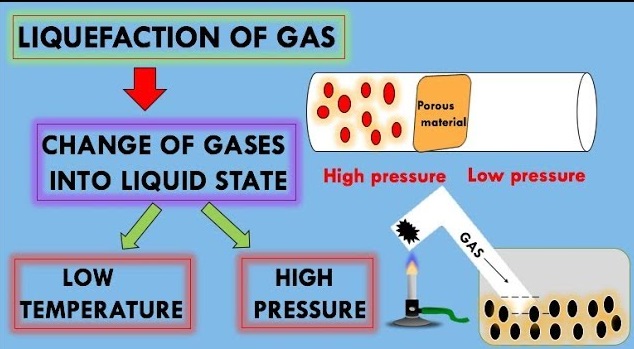
Liquefaction of Gases
For important commercial operations such as LPG and rocket fuels, we require gases in their liquid state. The liquefication methods are based on the Joule-Thomson effect. He observed appreciable cooling when the compressed gas is forced through an orifice plug into a low-pressure region. This phenomenon of lowering of temperature when a gas is made to expand adiabatically from a region of high pressure into a region of low pressure is known as JouleThomson effect.
This effect is observed only below a certain temperature, which is a characteristic one for each gas. This temperature below which a gas obeys Joule-Thomson effect is called inversion temperature (Ti). This value is given us van der waals constants a and b.
T1 = \(\frac{2a}{Rb}\) …………… (6.34)
Gases like O2, He, N2 and H2 have very low Tc, hence Joule-Thomson effect can be applied for cooling effectively. At the inversion temperature, no rise or fall in temperature of a gas occurs while expanding. But above the inversion temperature, the gas gets heated up when allowed to expand through a hole.
There are different methods used for liquefaction of gases:
1. In Linde’s method, Joule-Thomson effect is used to get liquid air or any other gas.
2. In Claude’s process, the gas is allowed to perform mechanical work in addition to Joule-Thomson effect so that more cooling is produced.
3. In Adiabatic process, cooling is produced by removing the magnetic property of magnetic material such as gadolinium sulphate. By this method, a temperature of 10-4 K i.e. as low as 0 K can be achieved.
Liquefaction of gases is physical conversion of a gas into a liquid state (condensation). The liquefaction of gases is a complicated process that uses various compressions and expansions to achieve high pressures and very low temperatures, using, for example, turboexpanders.
In gases, the intermolecular distance is very large. But when we apply high pressure and lower the temperature the gas converts to liquid. Thus, the most favourable conditions to liquefy a gas are high pressure and low temperature. Thus, the correct option is (B) low temperature and high pressure.
The critical temperature signifies the force of attraction between the molecules. The higher the critical temperature, higher is the intermolecular force of attraction and easier is the liquefaction of the gas. Gases require cooling and compression both for liquefaction.
The liquefaction of a gas takes place when the intermolecular forces of attraction become so high that they bind the gas molecules together to form the liquid state. For each gas, there is a particular temperature above which it cannot be liquefied, howsoever, high pressure may be applied on the gas.
Liquefaction takes place when loosely packed, water-logged sediments at or near the ground surface lose their strength in response to strong ground shaking. Liquefaction occurring beneath buildings and other structures can cause major damage during earthquakes.
In general, gases can be liquefied by one of three methods:
(1) By compressing the gas at temperatures less than its critical temperature
(2) By making the gas do some kind of work against an external force, which causes the gas to lose energy and change to the liquid state; and
(3) By making gas do work against its
The permanent gases have weak intermolecular forces of interaction which makes the process of liquefaction impossible to carry out. Since the options have hydrogen, oxygen and nitrogen, it is clear that they are permanent gases. Only chlorine can be liquified easily by applying the suitable pressure on it.
But, the only difference between Linde Claude’s process of liquefaction of air, or other gases is that in Claude’s process there is an isentropic expansion. That’s why Claude’s process is more efficient than Linde’s process.