Find free online Chemistry Topics covering a broad range of concepts from research institutes around the world.
Hess’s Law of Constant Heat Summation
We have already seen that the heat changes in chemical reactions are equal to the difference in internal energy (ΔU) or heat content (ΔH) of the products and reactants, depending upon whether the reaction is studied at constant volume or constant pressure.
Since ΔU and ΔH are functions of the state of the system, the heat evolved or absorbed in a given reaction depends only on the initial state and final state of the system and not on the path or the steps by which the change takes place.
This generalisation is known as Hess’s law and stated as:
The enthalpy change of a reaction either at constant volume or constant pressure is the same whether it takes place in a single or multiple steps provided the initial and final states are same.
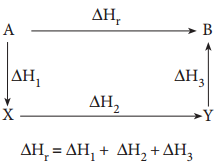
Application of Hess’s Law:
Hess’s law can be applied to calculate enthalpies of reactions that are difficult to measure. For example, it is very difficult to measure the heat of combustion of graphite to give pure CO.
However, enthalpy for the oxidation of graphite to CO2 and CO to CO2 can easily be measured. For these conversions, the heat of combustion values are – 393.5 kJ and – 283.5 kJ respectively. From these data the enthalpy of combustion of graphite to CO can be calculated by applying Hess’s law.
The reactions involved in this process can be expressed as follows
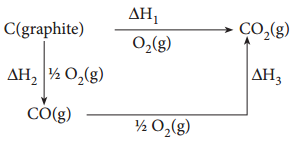
According to Hess law,
ΔH1 = ΔH2 + ΔH3
– 393.5 kJ = X – 283.5 kJ
X = – 110.5 kJ
Hess’s law, also called Hess’s law of constant heat summation or Hess’s law of heat summation, rule first enunciated by Germain Henri Hess, a Swiss-born Russian chemist, in 1840, stating that the heat absorbed or evolved (or the change in enthalpy) in any chemical reaction is a fixed quantity and is independent of the law.
The Hess’s law states that the total enthalpy change during a complete chemical reaction is the same regardless of the path taken by the chemical reaction. Hess’s law can be seen as an application of the principle of conservation of energy.
Hess’s law is useful to calculate heats of many reactions which do not take place directly. It is useful to find out heats of extremely slow reaction. It is useful to find out the heat of formation, neutralization, etc.
Hess’ law can be used to determine the overall energy required for a chemical reaction, when it can be divided into synthetic steps that are individually easier to characterize. This affords the compilation of standard enthalpies of formation, that may be used as a basis to design complex syntheses.
Hess law of constant heat summation states that the total enthalpy change during a reaction is the same whether the reaction takes place in one step or in several steps.
Application:
Hess law is useful to calculate heats of many reactions which do not take place directly.
To experimentally measure the ΔH values of two reactions using the technique of constant pressure calorimetry. To apply these ΔH values in a Hess’s Law calculation to determine the enthalpy of combustion of a metal.
By converting the methanol to formaldehyde and hydrogen the enthalpy of the fuel has been increased. When the formaldehyde and the hydrogen are burned 86 kJ more energy will be released than when methonol is burned H is per mole. means that for each mole of methanol burned 677 kJ heat are released.
Hess’s law is due to enthalpy being a state function, which allows us to calculate the overall change in enthalpy by simply summing up the changes for each step of the way, until product is formed. All steps have to proceed at the same temperature and the equations for the individual steps must balance out.
Germain Henri Hess is noted today for two fundamental principles of thermochemistry: the law of constant summation of heat (known simply as Hess’s law) and the law of thermoneutrality.
What is the connection between Hess’s Law and the fact that H is a state function? Hess’s Law is a consequence of the fact that enthalpy is a state function. Since ΔH is independent of path, we can describe a process by any series of steps that add up to the overall process.
Hess’s law states that the energy change in an overall chemical reaction is equal to the sum of the energy changes in the individual reactions comprising it.
Hess’s Law of Constant HeatSummation (or just Hess’s Law) states that regardless of the multiple stages or steps of a reaction, the total enthalpy change for the reaction is the sum of all changes. This law is a manifestation that enthalpy is a state function.