Find free online Chemistry Topics covering a broad range of concepts from research institutes around the world.
First Law of Thermodynamics
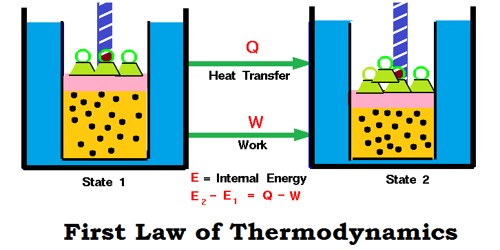
The first law of thermodynamics, known as the law of conservation of energy, states that the total energy of an isolated system remains constant though it may change from one form to another.
When a system moves from state 1 to state 2, its internal energy changes from U1 to U2. Then change in internal energy
∆U = U2 – U1.
This internal energy change is brought about by the either absorption or evolution of heat and/or by work being done by/on the system.
Because the total energy of the system must remain constant, we can write the mathematical statement of the
First Law as:
ΔU = q + w ………………… (7.7)
Where q – the amount of heat supplied to the system; w – work done on the system
Other statements of first law of thermodynamics
1. Whenever an energy of a particular type disappears, an equivalent amount of another type must be produced.
2. The total energy of a system and surrounding remains constant (or conserved)
3. “Energy can neither be created nor destroyed, but may be converted from one form to another”.
4. “The change in the internal energy of a closed system is equal to the energy that passes through its boundary as heat or work”.
5. “Heat and work are two ways of changing a system’s internal energy”.
Mathematical Statement of the First Law
The mathematical statement of the first law of thermodynamics is
ΔU = q + w ……………… (7.7)
Case 1:
For a cyclic process involving isothermal expansion of an ideal gas,
ΔU = 0.
eqn (7.7) ⇒ ∴ q = – w
In other words, during a cyclic process, the amount of heat absorbed by the system is equal to work done by the system.
Case 2:
For an isochoric process (no change in volume) there is no work of expansion. i.e. ΔV = 0
ΔU = q + w
= q – PΔV
ΔV = 0
ΔU = qV
In other words, during an isochoric process, the amount of heat supplied to the system is converted to its internal energy.
Case 3:
For an adiabatic process there is no change in heat. i.e. q = 0. Hence q = 0
eqn (7.7) ⇒ ΔU = w
In other words, in an adiabatic process, the decrease in internal energy is exactly equal to the work done by the system on its surroundings.
Case 4:
For an isobaric process. There is no change in the pressure. P remains constant. Hence
ΔU = q + w
ΔU = q – P ΔV
In other words, in an isobaric process a part of heat absorbed by the system is used for P-V expansion work and the remaining is added to the internal energy of the system.
Problem: 7.1
A gas contained in a cylinder fitted with a frictionless piston expands against a constant external pressure of 1 atm from a volume of 5 litres to a volume of 10 litres. In doing so it absorbs 400 J of thermal energy from its surroundings. Determine the change in internal energy of system.
Solution:
Given data q = 400 J V1 = 5L V2 = 10L
Δu = q – w (heat is given to the system (+q); work is done by the system(-w)
Δu = q – PdV
= 400 J – 1 atm (10-5)L
= 400 J – 5 atm L
[∴1L atm = 101.33 J]
= 400 J – 5 × 101.33 J
= 400 J – 506.65 J
= – 106.65 J