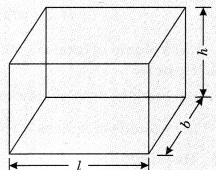
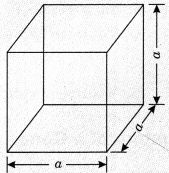
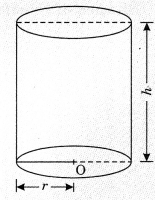
 On this page, you will find Chemical Reactions and Equations Class 10 Notes Science Chapter 1 Pdf free download. CBSE NCERT Class 10 Science Notes Chapter 1 Chemical Reactions and Equations will seemingly help them to revise the important concepts in less time.
On this page, you will find Chemical Reactions and Equations Class 10 Notes Science Chapter 1 Pdf free download. CBSE NCERT Class 10 Science Notes Chapter 1 Chemical Reactions and Equations will seemingly help them to revise the important concepts in less time.
CBSE Class 10 Science Chapter 1 Notes Chemical Reactions and Equations
Chemical Reactions and Equations Class 10 Notes Understanding the Lesson
1. Chemical reaction: The reaction in which the original state of the particles changes and it cannot be reversed by simple physical means, is known as a chemical reaction.
Examples: fermentation of grapes, burning of wood, etc. Burning of wood produces charcoal and we cannot get back wood from charcoal on reversing the conditions.
- Chemical reaction is accompanied by change in state, colour, evolution of gas or change in temperature. The chemical reaction is represented as
Reactants → Products - Example of a chemical reaction is burning of magnesium ribbon with a dazzling white flame to form a white powder (magnesium oxide).
2Mg + O2 → 2MgO
2. Chemical equation: Representation of a chemical reaction in terms of chemical symbols and formulae of the reactants and products is known as chemical equation. A chemical equation represents the reactants, products and their physical states symbolically.
For example,
Magnesium + Oxygen → Magnesium oxide
2Mg(s) + O2(g) → 2MgO(s)
The substances that undergo chemical change in the reaction, i.e., magnesium and oxygen, are the reactants. The new substance, magnesium oxide, formed during the reaction is the product.
Writing a chemical reaction in terms of chemical equation: A chemical equation represents a chemical reaction. While writing a chemical equation the reactants are written on the left hand side of the equation while products on the right hand side.
Examples:
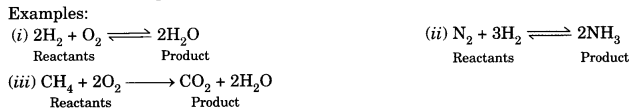
3. Balanced chemical equation: The chemical equation in which the number of atoms of different elements is same on both sides of the arrow is called a balanced chemical equation.
This is in accordance to the law of conservation of mass.
Let us try to balance the following chemical equation:
Fe + H2O → Fe3O4 + H2
Number of atoms of different elements present in the unbalanced equation
| Element | Number of atoms in reactants (LHS) | Number of atoms in products (RHS) |
| Fe | 1 | 3 |
| H | 2 | 2 |
| O | 1 | 4 |
Now select the element which has the maximum number of atoms Fe304. There are four oxygen atoms on the RHS and only one on the LHS.
To balance the oxygen atoms
| Atoms of oxygen | In reactants | In products |
| (i) Initial | 1 (in H2O) | 4 (in Fe3O4) |
| (ii) To balance | 1 x 4 | 4 |
So, multiplying H2O molecules by four, we get
Fe + 4H2O → Fe3O4 + H2
To balance H atoms, make the number of molecules of hydrogen as four on the RHS.
Fe + 4H2O → Fe3O4 + 4H2
To equalise Fe, we multiply Fe atoms by three on the LHS.
Hence,
3Fe + 4H2O → Fe3O4 + 4H2 (Balanced equation)
To make chemical equation more informative gaseous, liquid, aqueous and solid states of reactants and products are represented by the notations (g), (l), (aq) and (s) respectively.
Hence, 3Fe(s) + 4H2O(g) → Fe3O4(s) + 4H2(g)
4.Types of chemical reactions
(a) Combination reaction: The reactions in which two or more substances combine to form a new substance is called combination reaction.
For example,
- 2Mg(s) + O2 (fe)→ 2MgO(s)
- CaO(s) + H2O(l) → Ca(OH)2(aq)
(b) Decomposition reaction: The reaction in which a single compound breaks up into two or more simpler substances is called decomposition reaction. For example,
2Pb(NO3)2(s) → 2PbO(s) + 4NO2(g)+ O2(g)
The decomposition of a substance by passing electric current through it is known as electrolysis.
![]()
The decomposition of a substance on heating is known as thermal decomposition.
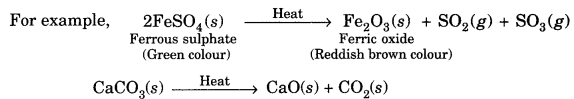
The decomposition of a substance by absorbing light energy is called photochemical decomposition.
For example,
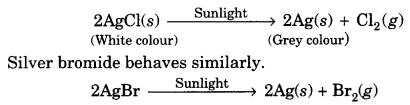
The above two reactions are used in black and white photography.
Decomposition reactions are opposite of combination reactions.
(c) Displacement reaction: The chemical reaction in which a more reactive element displaces a less reactive element from its salt solution is known as displacement reaction. For example,
- Zn(s) + CuSO4(aq)→ ZnSO4(aq) + Cu(s)
- Cu(s) + 2AgNO3(aq) → Cu(NO3)2 (aq) + 2Ag(s)
(d) Double displacement reaction: In this reaction two different atoms or group of atoms are mutually exchanged.
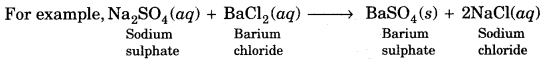
A white, insoluble substance, i.e., BaS04 is formed which is called precipitate.
Precipitation Reaction-Any reaction that produces a precipitate is called a precipitation reaction.
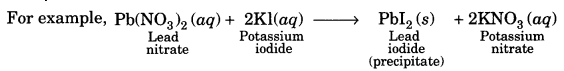
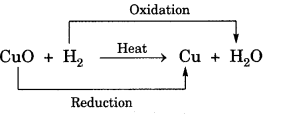
(e) Oxidation: Oxidation is the gain of oxygen or loss of hydrogen.
![]()
(f) Reduction: Reduction is the loss of oxygen or gain of hydrogen.
![]()
Redox reaction: The reaction in which one reactant gets oxidised while other gets reduced.
Exothermic reactions: Reaction in which heat is released along with the formation of products.
For example, \(\mathrm{CH}_{4}(g)+2 \mathrm{O}_{2}(g) \longrightarrow \mathrm{CO}_{2}(\mathrm{g})+2 \mathrm{H}_{2} \mathrm{O}(g)\)
Respiration and decomposition of vegetable matter into compost are exothermic reactions.
Endothermic reactions: The reactions which require energy in form of heat, light or electricity are called endothermic reactions.
\(2 \mathrm{Ba}(\mathrm{OH})_{2}+2 \mathrm{NH}_{4} \mathrm{Cl} \longrightarrow \text { Heat } \mathrm{2BaCl}_{2}+2 \mathrm{NH}_{4} \mathrm{OH}\)
5. Corrosion: The process of slow deterioration of some metals like iron, copper and silver into their compounds due to their reaction with oxygen, water, acids, gases, etc. present in the atmosphere is called corrosion.
Rusting The process in which iron reacts with oxygen and moisture present in the air to form a reddish brown coating called rust on its surface.
6. Rancidity: The taste and odour of food materials containing fat and oil changes when they are left exposed to air for a long time. This is called rancidity. It is caused due to oxidation of fat and oil present in food material. It can be prevented by using various methods such as by adding antioxidants to the food materials, storing food in air tight containers and by flushing out air with nitrogen.
Class 10 Science Chapter 1 Notes Important Terms
Chemical reaction is a process in which old bond breaks up and new bonds are formed.
Chemical equation is the representation of a chemical reaction in terms of chemical symbols and formulae.
Combination reaction is a reaction in which two or more substances combine to form a new substance.
Decomposition reaction is a reaction in which a single compound breaks up into two or more simpler substances.
Displacement reaction is a reaction in which a more reactive element displaces a less reactive element from its salt solution.
Redox reaction is the reaction in which oxidation and reduction takes place simultaneously.