On this page, you will find Life Processes Class 10 Notes Science Chapter 6 Pdf free download. CBSE NCERT Class 10 Science Notes Chapter 6 Life Processes will seemingly help them to revise the important concepts in less time.
On this page, you will find Life Processes Class 10 Notes Science Chapter 6 Pdf free download. CBSE NCERT Class 10 Science Notes Chapter 6 Life Processes will seemingly help them to revise the important concepts in less time.
CBSE Class 10 Science Chapter 6 Notes Life Processes
Life Processes Class 10 Notes Understanding the Lesson
1. Life Processes: All the processes like respiration, digestion, which together keep the living organisms alive and help in maintaining the functions of the body, are called life processes.
2. Nutrition: The process by which an organism obtains its food is called nutrition.
Modes of Nutrition:
- Autotrophic nutrition: Kind of nutrition in which inorganic materials like CO2, water, etc. are utilised to prepare organic food by the process of photosynthesis. For example, green plants and some bacteria.
- Heterotrophic nutrition: Kind of nutrition in which organisms do not possess the ability to synthesise their own food. They depend on autotrophs for their food supply directly or indirectly. For example, animals, fungi.
3. Bio-catalysts: Enzymes are called bio-catalysts as they play an important role in chemical reactions taking place in living organisms.
4. Photosynthesis: Autotrophs take in CO2 and H2O and convert these into carbohydrates in the presence of chlorophyll and sunlight by the process called Photosynthesis.
5. Equation of Photosynthesis:
![]()
6. Raw Materials for Photosynthesis:
- Sunlight
- Chlorophyll-It helps to trap the energy of the Sun
- CO2-It enters through Stomata
- Water-Water and dissolved minerals are taken up by the roots from soil
7. Products of Photosynthesis:
- Carbohydrates in the form of glucose
- Oxygen (O2)-released as a by-product
8. Site of Photosynthesis:
Chloroplast is the site of photosynthesis. Chloroplast contains a green pigment called chlorophyll which helps to trap energy of sunlight.
9. Events of Photosynthesis:
- Absorption of light energy by chlorophyll.
- Conversion of light energy to chemical energy + splitting of water molecules into hydrogen and oxygen.
- Reduction of carbon dioxide to carbohydrates.
10. Structure of Leaf:
External structure of leaf comprises of
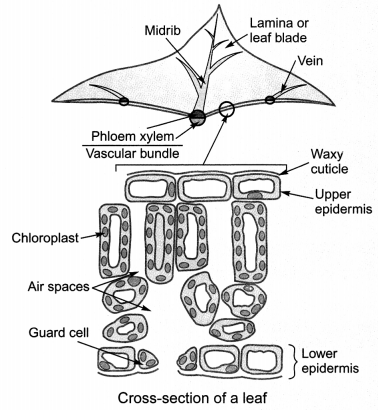
- Petiole: Stalk of leaf.
- Lamina: Flat, broad and expanded portion of leaf.
- Midrib: Midline which divides leaf into two equal halves.
- Veins: Supply water throughout surface of leaf.
11. Internal structure of leaf comprises of:
Epidermis: It has two parts:
(i) upper epidermis and
(ii) lower epidermis.
(i) Upper epidermis is usually covered by a waxy layer called cuticle which prevents water loss through transpiration.
(ii) Lower epidermis has stomata which help in gaseous exchange
Stomata: Tiny pores which are generally found in the lower epidermis and help in gaseous exchange and transpiration.
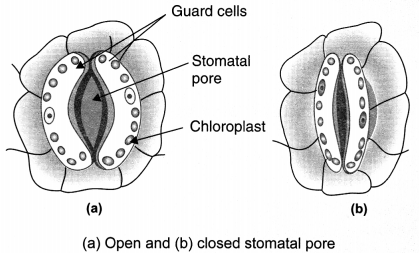
Guard cells: Bean-shaped cells which guard the opening of stomata. They have chloroplasts and have uneven thickening in their cell wall. Opening and closing of stomatal pore is done by guard cells. Movement of water into guard cells cause their swelling and open the stomatal pore. The shrinking of the guard cells when water moves out causes closing of stomatal pore.
12. Types of Heterotrophic Nutrition:
- Holozoic nutrition: The complex food material taken in by the organism is broken down into simpler and soluble molecules. For example, Human, Amoeba.
- Saprotrophic nutrition: The organisms feed on the dead and decaying matter. For example, Fungi.
- Parasitic nutrition: The organisms live either on or inside the organism to obtain its nutrition. For example, Lice, Cuscuta (amarbel).
13. Steps in Holozoic Nutrition:
- Ingestion
- Digestion
- Absorption
- Assimilation
- Egestion.
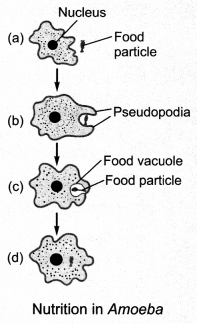
14. Nutrition in Amoeba
Temporary finger-like extensions of the cell surface called pseudopodia are used by Amoeba to engulf food. Pseudopodia fuse over the food particle forming a food vacuole in which complex substances are broken down into simpler ones and diffuse into the cytoplasm. The remaining undigested material moves to the surface of the cell and gets thrown out.
15. Nutrition in Paramoecium
In Paramoecium, food is moved to a specific spot by the movement of cilia which cover the entire surface of the cell.
16. Nutrition in Human Beings
Human digestive system consists of alimentary canal and the associated glands. The alimentary canal is a long tube extending from the mouth to the anus.
17. Mouth – Helps in intake of whole food.
18. Teeth – Helps in chewing and grinding of food.
19. Tongue – Helps in tasting food + rolling food + swallowing food.
20. Salivary glands – Secrete saliva and mucus. The enzyme called salivary amylase is present in saliva which breaks down the complex starch into sugar.
21. Oesophagus (food pipe) – Food moves towards stomach through oesophagus by rhythmic contraction of its muscles called peristaltic movements or peristalsis.
22. Stomach – Muscular walls of stomach help in mixing food thoroughly with digestive juices. Stomach has gastric glands which secrete gastric juice containing pepsin, hydrochloric acid and mucus.
- Pepsin helps in digestion of proteins.
- Hydrochloric acid creates an acidic medium which facilitates the action of the enzyme pepsin + kills germs present in food particles.
- Mucus protects the inner lining of the stomach from the action of the hydrochloric acid under normal conditions.
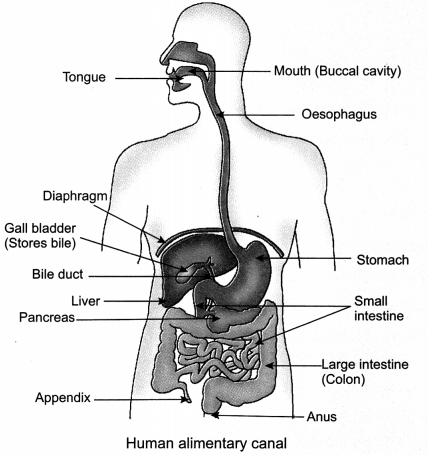
23. Small Intestine: A sphincter muscle regulates the exit of food from stomach into the highly coiled, longest part of the alimentary canal called the small intestine. Herbivores have a longer small intestine compared to the carnivores to allow the cellulose present in the grass to get digested. The digestive juices released in small intestine are:
(i) Bile juice: It is released from liver (stored in gall bladder). It helps to create alkaline medium in the small intestine for the pancreatic enzymes to act. Bile salts present in bile juice break down large fat globules into smaller globules (emulsification of fats) to increase the efficiency of enzyme action.
(ii) Pancreatic juice: It is released from pancreas and contains enzymes like trypsin for digesting proteins and lipase for breaking down emulsified fats.
(iii) Intestinal juice: The walls of the small intestine contain glands which secrete intestinal juice. The enzymes present in it finally convert the proteins to amino acids, complex carbohydrates into glucose and fats into fatty acids and glycerol. Intestinal juice completes the process of digestion.
(iv) Role of Villi: The digested food is absorbed by the inner lining or wall of the intestine with the help of villi. Villi are finger-like projections richly supplied with blood vessels and help to increase the surface area for absorption. Absorbed nutrients reach the cells through blood and are utilised for obtaining energy, building up new tissues and the repair of old tissues.
(v) Large Intestine: The unabsorbed food is sent into the large intestine where more villi absorb water from this material and remove the wastes through the anus by egestion. The exit of this waste material is regulated by the anal sphincter.
24. Respiration: The breakdown of simple food material to release energy is called as respiration. Aerobic respiration takes place in the presence of air (oxygen) whereas the anaerobic respiration occurs in the absence of air (oxygen). More amount of energy is released in aerobic respiration.
25. Glycolysis: This is the first step which occurs in the cytoplasm and results in breakdown of glucose (six-carbon molecule) into a three-carbon molecule called pyruvate. Glycolysis occurs both in aerobic as well as anaerobic respiration.
26. Fate of pyruvic acid (pyruvate):
- The pyruvate is converted into ethanol and carbon dioxide by the process called fermentation in yeast due to anaerobic respiration.
- The pyruvate is converted into a three-carbon compound lactic acid during respiration in muscle cells due to anaerobic respiration. Accumulation of lactic acid causes cramps in muscles.
- The pyruvate is broken down into carbon dioxide and water in presence of oxygen inside the mitochondria. The energy released during cellular respiration is used to synthesise a molecule called ATP which is used to fuel all other activities in the cell.
27. Types of Respiration:
(i) Aerobic
- Anaerobic respiration
- Aerobic respiration: It takes place in the presence air (oxygen).
Anaerobic respiration: It takes place in the absence of air (oxygen).
Respiration in plants: Respiration in plants is simpler than the respiration in animals.
28. Gaseous exchange occurs through:
- Stomata in leaves
- Lenticels in stems
- General surface of the roots.
29. Respiration in terrestrial animals: They use atmospheric oxygen for respiration.
30. Respiration in aquatic animals: Aquatic animals use the oxygen dissolved in water. They breathe at a faster rate since the amount of dissolved oxygen is fairly low compared to the amount of oxygen in the air. Fishes take water from mouth and send it to the gills where the dissolved oxygen is taken up by blood.
31. Human Respiratory System: Air enters the body after getting filtered by fine hairs and mucus in the nostrils. The air then passes through trachea (present in throat) into the lungs. Rings of cartilage present on trachea prevent it from collapsing during the passage of air.
The trachea divide into bronchi which enter the lungs and divide further into bronchioles which finally terminate in balloon-like structures called alveoli which have a rich supply of blood vessels and help in exchange of gases.
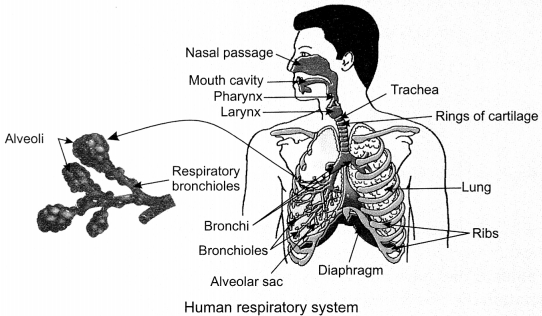
32. Mechanism of breathing: During inhalation (breathing in), the volume of the chest cavity becomes larger as the ribs get lifted and diaphragm gets flattened. Air gets sucked into the lungs and fills the expanded alveoli. The blood brings carbon dioxide from the rest of the body to the alveoli and exchanges it for oxygen to be transported to all the cells in the body.
During exhalation (breathing out), the volume of the chest cavity becomes smaller as the ribs get relaxed and diaphragm moves upward (relaxes). Air rich in carbon dioxide gets pushed out of the lungs to come out through the nostrils.
33. Residual volume: It is the volume of air left behind in the lungs even after forceful breathing out of air. This helps to provide sufficient time for oxygen to be absorbed and for the carbon dioxide to be released.
34. Respiratory pigment: The respiratory pigment called haemoglobin in human beings is present in the red blood corpuscles. Haemoglobin has a very high affinity for oxygen.
35. Transportation
Transportation in Human Beings: Circulatory system helps to transport blood to various parts of the body to ensure the supply of nutrients and oxygen to these parts and remove carbon dioxide and metabolic wastes.
The circulatory system in human beings consists of:
- A pumping organ—a muscular heart
- Blood vessels—Arteries and veins
- Circulating medium—Blood and lymph
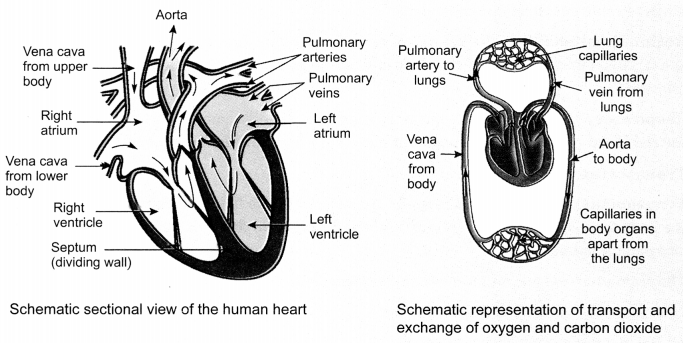
36. Steps in circulation of blood:
- The relaxed thin-walled upper chamber of the heart on the left, the left atrium, receives oxygen- rich blood from the lungs through the pulmonary vein.
- The left atrium contracts and transfers blood to the left ventricle.
- The left ventricle contracts and sends the oxygen-rich blood through aorta to the various parts of the body.
- De-oxygenated blood from the various parts of the body is transported by vena cava to the relaxed right upper chamber of the heart called the right atrium.
- The right atrium contracts and transfers blood to the right ventricle.
- The right ventricle pumps de-oxygenated blood for oxygenation to the lungs through pulmonary vein.
- Ventricles have thicker muscular walls than atrium as they have to pump blood into various organs.
37. Role of valves: Valves ensure that blood does not flow backwards when the atria or ventricles contract.
Significance of separation of right and left side of the heart: It is useful to prevent mixing of oxygenated and de-oxygenated blood. It also allows a highly efficient supply of oxygen to the body. It is useful for animals that have high energy needs, such as birds and mammals, which constantly use energy to maintain their body temperature.
38. Types of heart:
Fishes have a two chambered heart. Blood pumped by heart gets oxygenated by gills and passes directly to the rest of the body. This is called single circulation. Amphibians and reptiles have three- chambered hearts and tolerate some mixing of the oxygenated and de-oxygenated blood. Birds and mammals have four chambered heart. Blood goes through the heart twice during each cycle in them. This is known as double circulation.
Types of Blood Vessels
| Arteries | Veins |
| (i) Carry blood from heart to various organs of the body. | (i) Carry blood from various organs of body to the heart. |
| (ii) Carry oxygenated blood from the heart except the pulmonary artery. | (ii) Carry de-oxygenated blood from various organs except the pulmonary vein. |
| (iii) Have thick and elastic walls. | (iii) Have thin non-elastic walls. |
| (iv) Valves are absent. | (iv) Valves are present. |
| (v) Blood flows under high pressure. | (v) Blood flows under low pressure. |
39. Capillaries: The smallest vessels have walls which are one-cell thick and are called capillaries. Their thin wall helps in exchange of material between the blood and surrounding cells. Veins are formed when the capillaries join together.
40. Role of blood platelets: Platelet cells circulate around the body in the blood and help in the clotting of blood when blood flows out during injury or cut.
41. Lymph or Tissue fluid: It is formed by the plasma, proteins and blood cells which escape into the intercellular spaces in the tissues through the pores present in the walls of the capillaries. Lymph is similar to the plasma of blood but colourless and contains less protein. It also carries digested and absorbed fat from intestine and drains excess fluid from extra cellular space back into the blood. Lymph enters the lymphatic capillaries which join to form large lymph vessels that finally open into larger veins.
42. Transportation in Plants: Two main conducting pathways in plants are:
(i) Xylem and
(ii) Phloem
(i) Xylem: It carries water and minerals from the roots to other parts of the plants.
(ii) Phloem: It carries products of photosynthesis from leaves to the other parts of the plant.
43. Transport of Water and Minerals
(i) By root pressure: The cells of root in contact with soil actively take up ions which creates a difference in ion concentration between the root and the soil. Water moves into the root from the soil to eliminate this difference, creating a column of water that is steadily pushed upwards.
(ii) By transpiration pull: Loss of water from stomata by transpiration gets replaced by the xylem vessels in the leaf which creates a suction to pull water from the xylem cells of the roots. This strategy is used during day time and helps to transport water to the highest points of the plant body.
44. Transpiration and its roles: The loss of water in the form of vapour from the aerial parts of the plant is known as transpiration.
It helps in
(i) absorption and upward movement of water and minerals.
(ii) temperature regulation by cooling the leaf surface.
45. Transport of food and other substances: Translocation is the transport of soluble products of photosynthesis through phloem.
Sucrose is transferred into sieve tubes of phloem via the companion cells using energy from ATP. This increases the osmotic pressure inside the sieve tubes which causes movement of water into the sieve tubes from the adjacent xylem. This pressure helps in translocation of material in the phloem to tissues which have less pressure.
46. Excretion: Removal of metabolic wastes from the body is called as excretion.
47. Excretion in Unicellular organisms: Many unicellular organisms remove metabolic wastes from the body surface into the surrounding water by simple diffusion.
48. Excretion in Human Beings: Excretory system of human beings includes:
(i) A pair of Kidneys
(ii) A Urinary Bladder
(iii) A pair of Ureters
(iv) A Urethra The purpose of making urine is to filter out waste product from the blood i.e., urea which is produced in the liver. Each kidney has large numbers of filtration units called nephrons. The urine formation involves three steps.
(i) Glomerular filtration: Nitrogenous wastes, glucose water, amino acid filter from the blood in blood capillaries into Bowman Capsule of the nephrons.
(ii) Selective reabsorption: Some substances in the initial filtrate, such as glucose, amino acids, salts and a major amount of water are selectively reabsorbed back by capillaries surrounding the nephrons.
(iii) Tubular secretion: Some ions like K+, H+, etc. are secreted into the tubule which opens up into the collecting duct.
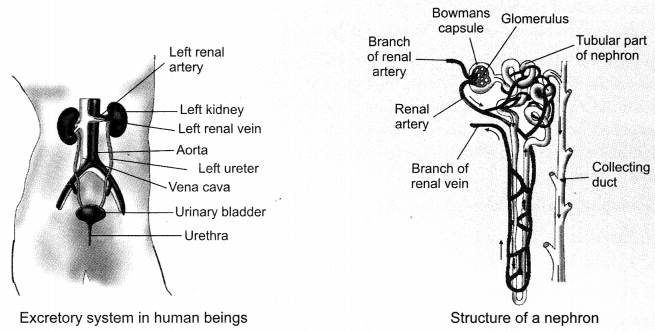
Urine produced in the kidneys passes through collecting duct into the ureters. Ureters takes urine into the urinary bladder where it is stored until it is released through the urethra. Release of urine is under nervous control.
49. Excretion in Plants: Excess oxygen and carbon dioxide removed through stomata. Excess water removed by transpiration through stomata.
Plant waste products are also removed by:
- Storage in cellular vacuoles
- Storage in leaves that fall off
- Storing as resins and gums in old xylem
- By excreting into the soil around them.
Class 10 Science Chapter 6 Notes Important Terms
Autotrophs: The organisms which can prepare their own food from inorganic substances by using light or chemical energy. For example, Green plants and Bacteria.
Heterotrophs: Organisms which cannot synthesise their own food and depend oh other organisms for their nutrition. For example, Humans.
Bio-catalysts: Enzymes are called as bio-catalysts as they play an important role in chemical reactions taking place in living organisms.
Photosynthesis: The process by which the green plants make their own food in the presence of carbon dioxide, water, chlorophyll and sunlight. The site of photosynthesis is chloroplast present in plant cells.
Ingestion: The process of taking in food into body of an organism.
Digestion: It is the breakdown of large insoluble food molecules into simpler and small water- soluble food molecules so that they can be absorbed into the blood stream.
Absorption: The process of passing the nutrients from the digested food into the blood stream from the walls of the intestine.
Assimilation: The process by which the absorbed food is used by the various cells and tissues of the body for growth and repair.
Egestion: The act or process of throwing out the undigested material from a cell or an organism is called as egestion.
Peristalsis: It is a series of wave-like muscle contractions of the muscles of the oesophagus that moves food into the stomach.
Emulsification: The breakdown of large fat globules in the duodenum into smaller globules in order to provide a larger surface area for the enzyme lipase to act and digest the fats into fatty acids and glycerol.
Inhalation: The process by which we take in air is called as inhalation. During inhalation, rib cage is moved up, diaphragm contracts (tightens) and moves downward to increase volume of chest cavity.
Exhalation: The process by which the air flows out of lungs is called as exhalation. During exhalation, the ribs relax; diaphragm relaxes and moves upward to reduce the volume of chest cavity.
Residual volume: The volume of air remaining in the lungs even after forceful exhalation is called residual volume.
Respiration: It is the biochemical process in which the cells of an organism obtain energy by combining oxygen and glucose, resulting in the release of carbon dioxide, water, and energy in the form of ATP.
Fermentation: It is a metabolic process which occurs under anaerobic conditions in yeast and bacteria to convert sugar to acids, gases or alcohol.
Haemoglobin: The respiratory pigment which helps to transport oxygen to various cells and tissues of the body.
Blood pressure: The force exerted by blood against the wall of a vessel is called blood pressure. It is measured by sphygmomanometer.
Translocation: It is the movement of materials from leaves to other tissues throughout the plant.
Transpiration: It is the evaporative loss of water by plants.
Nephron: It is the functional unit of kidney. It has two parts—the glomerulus and the renal tubule.
Haemodialysis: This is a process which helps in purifying the blood of a patient suffering from kidney problem or failure, using an artificial kidney. It removes nitrogenous waste products from the blood through dialysis.
 On this page, you will find Periodic Classification of Elements Class 10 Notes Science Chapter 5 Pdf free download. CBSE NCERT
On this page, you will find Periodic Classification of Elements Class 10 Notes Science Chapter 5 Pdf free download. CBSE NCERT 
 On this page, you will find Carbon and its Compounds Class 10 Notes Science Chapter 4 Pdf free download. CBSE NCERT
On this page, you will find Carbon and its Compounds Class 10 Notes Science Chapter 4 Pdf free download. CBSE NCERT 














 On this page, you will find Metals and Non-metals Class 10 Notes Science Chapter 3 Pdf free download. CBSE NCERT
On this page, you will find Metals and Non-metals Class 10 Notes Science Chapter 3 Pdf free download. CBSE NCERT 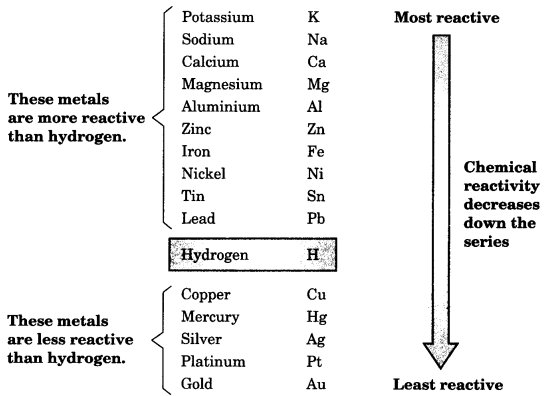
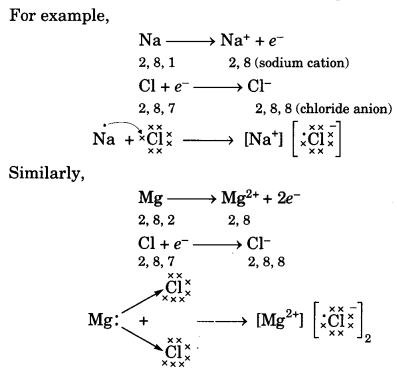
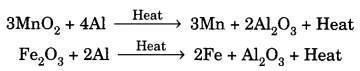
 On this page, you will find Acids Bases and Salts Class 10 Notes Science Chapter 2 Pdf free download. CBSE NCERT
On this page, you will find Acids Bases and Salts Class 10 Notes Science Chapter 2 Pdf free download. CBSE NCERT