Learninsta presents the core concepts of Microbiology with high-quality research papers and topical review articles.
Electron Transport Chain
An electron transport chain consists of a sequence of carrier molecules that are capable of oxidation and reduction. In and FADH2 to acceptor such as molecular Oxygen. In the process, protons are pumped from the mitochondrial matrix to the inner membrane space, and eventually combine with O2 and H+ to form water (Figure 4.6).
As the electrons flow through the chain, much of their free energy is conserved in the form of ATP. The process by which energy from electron transport is used to make ATP is called as oxidative phosphorylation.
Respiratory chain is an electron transport chain where a pair of electrons or hydrogen atoms containing electron from the substrate oxidized is coupled to reduction of oxygen to water.
The mitochondrial system is arranged Eukaryotic cell, the ETC is contained in the inner membrane of mitochondria or chloroplast membrane, whereas in prokaryotic cells, it is found in plasma membrane or cytoplasmic membrane.
The ETC is carried out through a series of electron transporters embedded in the inner mitochondrial membrane that transfer electrons from electron donors NADH into three complexes of electron carriers.
They are:
1. Flavoproteins:
These proteins contain flavin, a coenzyme derived from riboflavin (Vit B12). One important flavoprotein is flavin mono nucleotide.
2. Ubiquinones (coenzyme Q):
These are small non protein carriers.
3. Cytochromes:
These are proteins with iron containing group, capable of existing alternately as reduced (Fe2+) and oxidized form (Fe3+). Cytochromes involved in ETC include cyt (b),cyt c1, cyt c, cyt a, cyt a3.
The first step in electron transport chain is the transfer of high energy electrons from NADH to FMN. This transfer actually involves the passage of hydrogen atom with 2e– to FMN, which then picks up an additional H+ from the surrounding aqueous medium.
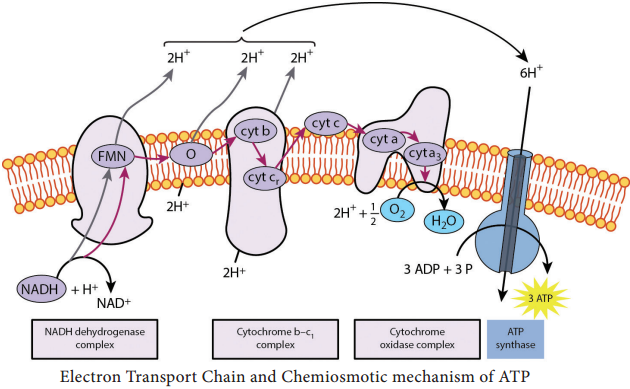
As a result of the first transfer, NADH is oxidized to NAD+, and FMN is reduced to FMNH2.
In the second step, FMNH2 passes 2 H+ to the other side of the mitochondrial membrane and passes 2 e– to coenzyme Q. As a result, FMNH2 is oxidized to FMN. Coenzyme Q also picks up additional 2H+ from the surrounding aqueous and releases to other side of the membrane.
In the next step, electrons are passed successively from coenzyme Q to cyt b1, cyt c1, cyt c, cyt a, cyt a3.
Each cytochrome in the chain is reduced, as it picks up electrons and is oxidized as it gives up electrons. The last cytochrome cyt a3 passes its electrons to molecular O2 which picks up protons from the surrounding medium to form H2O.
FADH2 derived from the Krebs cycle is another source of electrons. Thus at the end of ETC, NADH pumps three protons (synthesizes 3ATPs) whereas FADH2 pumps only two protons (synthesizes 2ATPs).
Chemiosmotic Mechanism of ATP
Chemiosmotic mechanism of ATP synthesis was first proposed by the Biochemist, Peter Mitchell in 1961. In ETC, when energetic electrons from NADH pass down the carriers, some of the carriers (proton pumps) in the chain pump [actively transport] protons across the membrane to inner membrane space.
Thus in addition to a concentration gradient, an electrical charge gradient is created. The resulting electro chemical gradient has potential energy called proton motive force.
The proton diffuses across the membrane through protein channels that contain an enzyme called ATP synthase. When this flow occurs, energy is released and is used by the enzyme to synthesize ATP from ADP and phosphate.
At the end of the chain, electrons join with protons and O2 in the matrix fluid to form H2O. Thus O2 is the final electron acceptor. ETC also operates in photophosphorylation and is located in thylakoid membrane of Cyanobacteria (BGA), and of eukaryotic chloroplasts. Overview of Aerobic respiration (Figure 4.7):
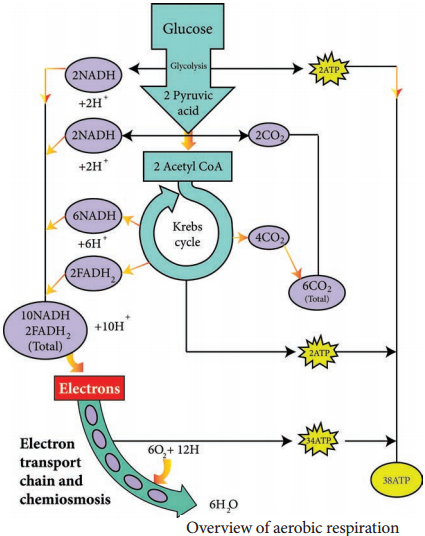
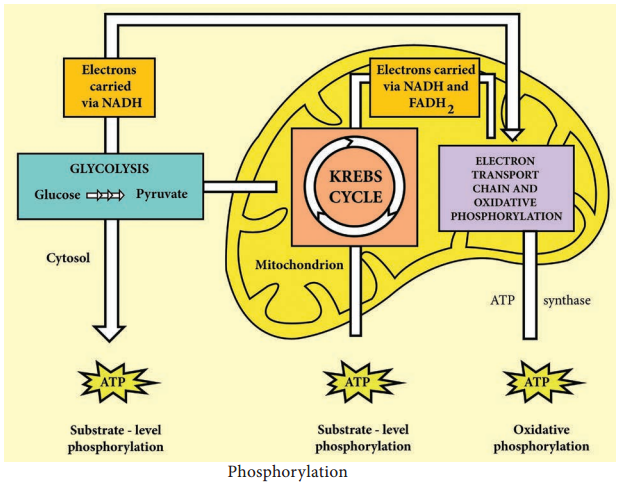
1. Electron transport chain regenerates NAD and FAD which can be used again in Glycolysis and Krebs cycle.
2. Various electrons transfer in the electron transport chain generates about 34 ATP, (10 NADH = 10 × 3 = 30 + 2 FADH2 = 2 × 2 = 4).
3. A total of 38 ATP molecules is generated from one molecule of glucose oxidized in prokaryotes, whereas in eukaryotes, 36 molecules of ATP is generated because in eukaryotes, some energy is lost when electrons are shuttled across the mitochondrial membranes that separate Glycolysis (in the cytoplasm) from the electron transport chain (Table 4.2). There is no such separation exists in prokaryotes.
C6H12O6 + 6CO2 + 38ADP + 38Pi → 6CO2 + 6H2O + 38 ATP
| Glycolysis 1. Oxidation of glucose to Pyruvic acid.
2. Production of 2 NADH | Preparatory step
2 ATP (substrate level phosphorylation)
6 ATP (Oxidative phosphorylation in ETC) |
| Preparatory step 1. Formation of acetyl CoA produces 2NADH | 6 ATP (Oxidative phosphorylation in ETC) |
| Krebs cycle 1. Oxidation of succinyl CoA to succinic acid
2. Production of 6 NADH
3. Production of 2 FADH | 2 ATP (Substrate level phosphorylation)
18 ATP (Oxidative phosphorylation in ETC)
4 ATP (Oxidative phosphorylation in ETC)
Total 38 ATP |
1 NADH = 3 ATPs and 1 FADH2 = 2 ATP