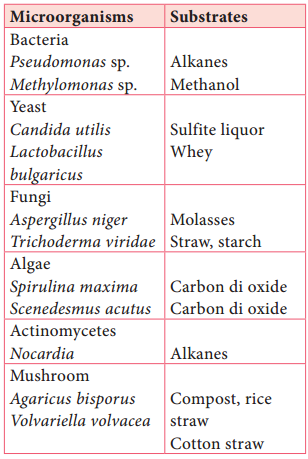
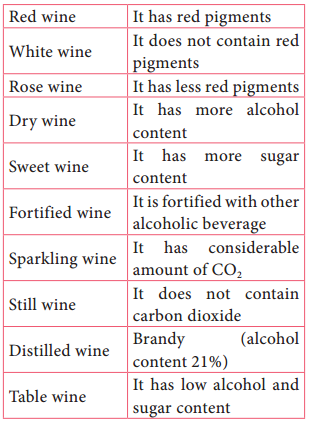
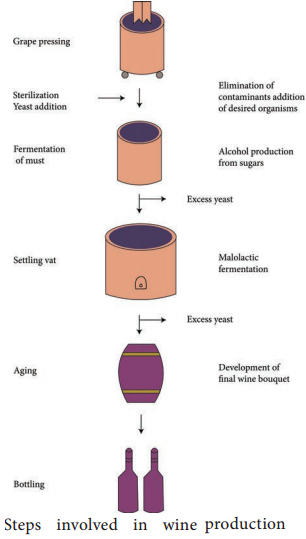
Learninsta presents the core concepts of Microbiology with high-quality research papers and topical review articles.
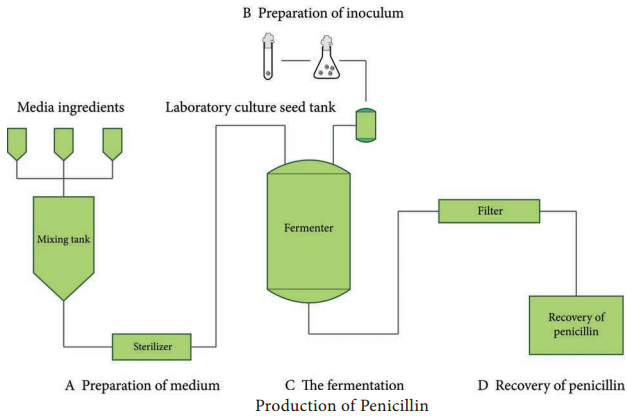
Industrial Production of Penicillin
Penicillin is a broad spectrum antibiotic. Penicillin is first obtained from the mould, Penicillium notatum (Figure 6.8).
Penicillium chrysogenum is a high yielding strain, used for the commercial production of penicillin. This strain is highly unstable, so the spore suspensions are maintained in a dormant state to prevent contamination. Most penicillin form filamentous broth and hence is difficult to mix and it hinders
oxygen transfer due to their high viscosity.
This is avoided by using bubble columns air lift reactors which agitates the medium providing even oxygen distribution. Penicillin has a basic structure 6-amino penicillanic acid β-(APA). It consists of a thiazolidine ring with a condensed β-lactum ring. It carries a variable side chain in position 6. Natural penicillins are produced in a fermentation process without adding any side chain precursors.
If a side chain precursor is added to the broth, desired penicillin is produced and it is called biosynthetic penicillin. Semi synthetic penicillin is one in which, both fermentation and chemical approach are used to produce useful pencillins. It can be taken orally and active against gram negative bacteria. (eg) Amphicilin. Nowadays, semi synthetic pencillins makeup the bulk of the penicillin market.
The initial strain of Penicillium chrysogenum (NRRL, 1951) was low yielding strain and so it is was treated with mutagenic agents such as X-rays, UV right and some other repeated methods to get a high yielding strain Q-176.
Production methods
Penicillin production is done by one of the following.
- Surface culture
- Submerged fermentation process
Inoculum Production
Inoculation methods
To inoculate fermentation medium one of the following methods can be employed.
- Using dry spores to seed the fermentation medium.
- Making suspension along with non toxic wetting agent like Sodium lauryl sulphate and inoculating germinated organism
- Using pellet inocula obtained by the germination of spores
The lyophilized spores (or) spores in well sporulated frozen agar slant are suspended in water or in a dilute solution of a nontoxic wetting agent.
(1: 10,000 sodium lauryl sulphonate)
↓
Spores are then added to a bottles containing wheat bran solution It is incubated for 5-7 days at 24°C for heavy sporulation.
↓
The resulting spores are then transferred to production tank
↓
The micro organism in the inoculum tank is checked for contamination.
Production process
The production tanks are inoculated with a mycelial growth.
↓
Production medium contains following medium components.
Carbon source as Lactose, Nitrogen source as Ammonium sulphate, Acetate or Lactate (Corn steep liquor is the cheap and easy source of nitrogen)
Mineral sources as K, P (Potassium di hydrogen phosphate), Mg, S (Magnesium sulphate), Zn, Cu(Copper sulphate) (Corn steep liquor supply some of these minerals)
Precursor (Example: phenyl acetic acid) is added to the medium
↓
Antifoam agent (Example: corn or soybean oil) is added before sterilization.
The sufficient aeration and agitation is given and are incubated at 25°C to 26°C for 3 to 5 days at PH range of 7 to 7.5
Penicillin Production
Process of penicillin production occurs in three phases:
First phase:
Growth of mycelium occurs in this phase where the yield of antibiotic is low. The pH increases due to the release of NH<sub>3</sub>.
Second phase:
In this phase, intense synthesis of penicillin occurs due to rapid consumption of Lactose and Ammonium nitrogen. The mycelial mass increases and the pH remain unchanged (Figure 6.10).
Third phase:
In this phase, the concentration of antibiotics decreases in the medium. Autolysis of mycelium starts, liberating Ammonia leading to slight rise in pH.
Recovery
After penicillin fermentation, the broth is filtered on rotary vacuum filter
↓
Mycelium is separated
↓
To the both sulphuric acid or phosphoric acid is added
↓
Pencilin is converted into anionic form
↓
It is extracted in counter current solvent extractor, by using organic solvent, amyl acetate, methyl isobutyl (ketone)
↓
It is then back extracted with water from the organic solvent by adding potassium or sodium hydroxide
↓
Shifts between water and solvent aid in the potassium or sodium hydroxide
↓
Shifts between water and solvent aid in the purification of pencilin
↓
The resulting sodium or potassium pencillin is then crystallized
↓
Then it is washed, dried and used for commercial purpose.