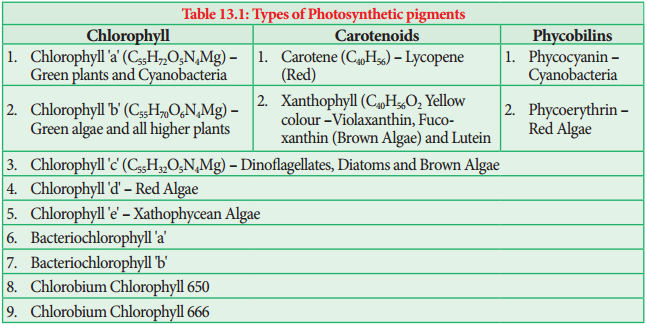
Learninsta presents the core concepts of Biology with high-quality research papers and topical review articles.
Spectrum of Electromagnetic Radiation
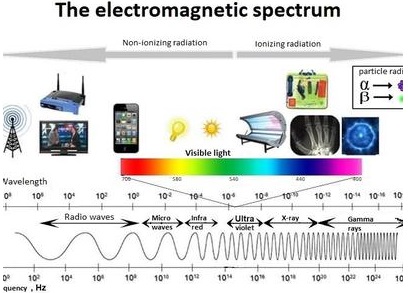
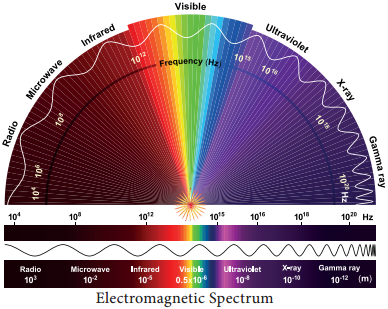
In the total electromagnetic spectrum, visible light is the smallest part. The entire life on earth depends on light and is the driving force for all organisms. Plants have natural potential to utilize solar energy directly. In the given picture electromagnetic radiation spectrum and components of visible spectrum are mentioned. The wavelength of solar radiation which reaches the earth is between 300 to 2600 nm.
The visible spectrum ranges between 390 to 763 nm (3900 å to 7630 å). The colour of the light is determined by the wavelength. Energy of the quantum is inversely proportional to wavelength. Shorter wavelength has more energy than longer wavelength. Electromagnetic spectrum consists of 7 types of radiations such as gamma rays, X rays, U-V rays, Visible light spectrum, infrared rays, electric rays and radio rays (Figure 13. 4).
Properties of Light
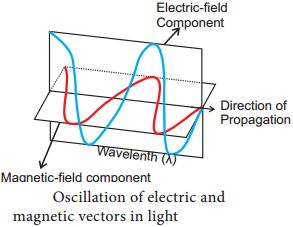
- Light is a transverse electromagnetic wave.
- It consists of oscillating electric and magnetic fields that are perpendicular to each other and perpendicular to the direction of propagation of the light.
- Light moves at a speed of 3 × 108 ms-1
- Wavelength is the distance between successive crests of the wave.
- Light as a particle is called photon. Each photon contains an amount of energy known as quantum.
- The energy of a photon depends on the frequency of the light (Figure 13.5).
The entire electromagnetic spectrum, from the lowest to the highest frequency (longest to shortest wavelength), includes all radio waves (e.g., commercial radio and television, microwaves, radar), infrared radiation, visible light, ultraviolet radiation, X-rays, and gamma rays.
The EM spectrum is generally divided into seven regions, in order of decreasing wavelength and increasing energy and frequency. The common designations are: radio waves, microwaves, infrared (IR), visible light, ultraviolet (UV), X-rays and gamma rays.
The electromagnetic spectrum is a continuum of all electromagnetic waves arranged according to frequency and wavelength. The sun, earth, and other bodies radiate electromagnetic energy of varying wavelengths. Electromagnetic energy passes through space at the speed of light in the form of sinusoidal waves.
The characteristics of the electromagnetic spectrum are the propagation features and the amount of information, which signals can carry. In general, signals sent using the higher frequencies have shorter propagation distances but a higher data-carrying capacity.
Radio waves, microwaves, visible light, and x rays are all examples of electromagnetic waves that differ from each other in wavelength.
- Longer Wavelength
- Shorter Wavelength
Electromagnetic waves are produced by the motion of electrically charged particles. The different types of waves have different uses and functions in our everyday lives. The most important of these is visible light, which enables us to see. Radio waves have the longest wavelengths of all the electromagnetic waves. They range from around a foot long to several miles long.
“Electromagnetic spectrum” refers to the spectrum of electromagnetic radiation, and electromagnetic radiation is so named because it consists of electric and magnetic fields. In fact, light does affect charges and currents.
These observations enable astronomers to determine certain physical characteristics of objects, such as their temperature, composition and velocity. The electromagnetic spectrum consists of much more than visible light. It includes wavelengths of energy that human eyes can’t perceive.
Cell phones use antennae to transmit and receive radio waves that carry binary information. Every cell tower presides over an area of land, where it receives and transmits radio waves. When a text message is written, it is transmitted as binary code using a particular frequency of radio waves specific to that user.
Almost all of the energy available at Earth’s surface comes from the sun. The sun gets its energy from the process of nuclear fusion. This energy eventually makes its way to the outer regions of the sun and is radiated or emitted away in the form of energy, known as electromagnetic radiation.
Yes, all objects, including human bodies, emit electromagnetic radiation. The wavelength of radiation emitted depends on the temperature of the objects. Such radiation is sometimes called thermal radiation. Most of the radiation emitted by human body is in the infrared region, mainly at the wavelength of 12 micron.