Learninsta presents the core concepts of Biology with high-quality research papers and topical review articles.
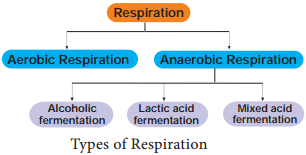
Stages of Respiration – Definition, Phases, Flow Chart
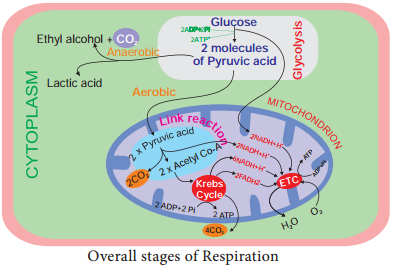
- Glycolysis-conversion of glucose into pyruvic acid in cytoplasm of cell.
- Link reaction-conversion of pyruvic acid into acetyl coenzyme-A in mitochondrial matrix.
- Krebs cycle-conversion of acetyl coenzyme A into carbon dioxide and water in the mitochondrial matrix.
- Electron transport chain to tranfer electrons remove hydrogen ions and transfer electrons from the products of glycolysis, link reaction and Krebs cycle.
- It takes place in mitochondrial inner membrane to release ATP with water molecule by terminal oxidation (Figure 14.5).
Glycolysis
(Gr: Glykos 5 Glucose, Lysis 5 Splitting) Glycolysis is a linear series of reactions in which 6-carbon glucose is split into two molecules of 3-carbon pyruvic acid. The enzymes which are required for glycolysis are present in the cytoplasm (Figure 14.6).
The reactions of glycolysis were worked out in yeast cells by three scientists Gustav Embden (German), Otto Meyerhoff (German) and J Parnas (Polish) and so it is also called as EMP pathway. It is the first and common stage for both aerobic and anaerobic respiration. It is divided into two phases.
- Preparatory phase or endergonic phase or hexose phase (steps 1-5).
- Pay off phase or oxidative phase or exergonic phase or triose phase (steps 6-10).
1. Preparatory Phase
Glucose enters the glycolysis from sucrose which is the end product of photosynthesis. Glucose is phosphorylated into glucose-6-phosphate by the enzyme hexokinase, and subsequent reactions are carried out by different enzymes (Figure 14.6). At the end of this phase fructose-1, 6-bisphosphate is cleaved into glyceraldehyde-3-phosphate and dihydroxy acetone phosphate by the enzyme aldolase.
These two are isomers. Dihydroxy acetone phosphate is isomerised into glyceraldehyde-3-phosphate by the enzyme triose phosphate isomerase, now two molecules of glyceraldehyde 3 phosphate enter into pay off phase. During preparatory phase two ATP molecules are consumed in step-1 and step-3 (Figure 14.6).
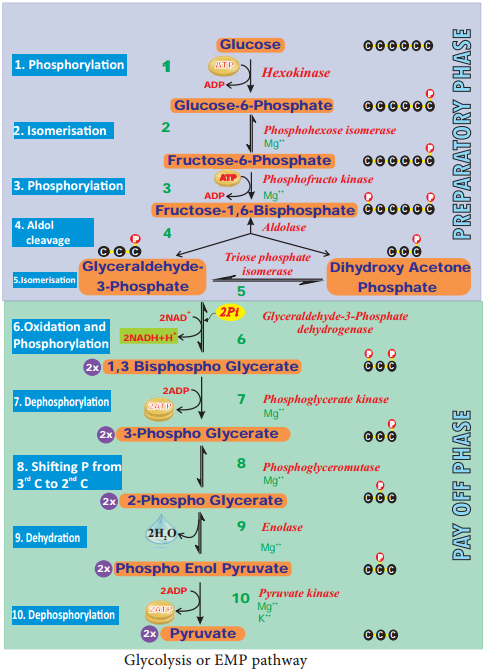
Pay Off Phase
Two molecules of glyceraldehyde-3-phosphate oxidatively phosphorylated into two molecules of 1,3 – bisphospho glycerate. During this reaction 2NAD+ is reduced to 2NADH+H+ by glyceraldehyde-3-phosphate dehydrogenase at step 6. Further reactions are carried out by different enzymes and at the end two molecules of pyruvate are produced.
In this phase, 2ATPs are produced at step 7 and 2 ATPs at step10 (Figure 14.6). Direct transfer of phosphate moiety from substrate molecule to ADP and is converted into ATP is called substrate phosphorylation or direct phosphorylation or trans phosphorylation. During the reaction at step 9, 2 phospho glycerate dehydrated into Phospho enol pyruvate. A water molecule is removed by the enzyme enolase. As a result, enol group is formed within the molecule. This process is called Enolation.

3. Energy Budget
In the pay off phase totally 4ATP and 2NADH + H+ molecules are produced. Since 2ATP molecules are already consumed in the preparatory phase, the net products in glycolysis are 2ATPs and 2NADH + H+.
The Overall Net Reaction of Glycolysis
C6H12O6 + 2ADP + 2Pi + 2NAD+
↓
2x CH3COCOOH + 2ATP + 2NADH + 2H+
Pyruvate Oxidation (Link reaction)
Two molecules of pyruvate formed by glycolysis in the cytosol enters into the mitochondrial matrix. In aerobic respiration this pyruvate with coenzyme A is oxidatively decarboxylated into acetyl CoA by pyruvate dehydrogenase complex. This reaction is irreversible and produces two molecules of NADH + H+ and 2CO2. It is also called transition reaction or Link reaction. The reaction of pyruvate oxidation is Pyruvate.

Krebs Cycle or Citric Acid Cycle or TCA Cycle:
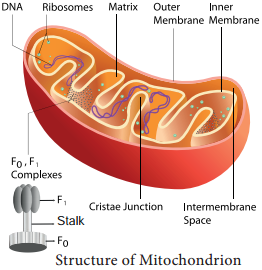
Two molecules of acetyl CoA formed from link reaction now enter into Krebs cycle. It is named after its discoverer, German Biochemist Sir Hans Adolf Krebs (1937). The enzymes necessary for TCA cycle are found in mitochondrial matrix except succinate dehydrogenase enzyme which is found in mitochondrial inner membrane (Figure 14.7).
TCA cycle starts with condensation of acetyl CoA with oxaloacetate in the presence of water to yield citrate or citric acid. Therefore, it is also known as Citric Acid Cycle (CAC) or Tri Carboxylic Acid (TCA) cycle. It is followed by the action of different enzymes in cyclic manner.
During the conversion of succinyl CoA to succinate by the enzyme succinyl CoA synthetase or succinate thiokinase, a molecule of ATP synthesis from substrate without entering the electron transport chain is called substrate level phosphorylation.
In animals a molecule of GTP is synthesized from GDP + Pi. In a coupled reaction GTP is converted to GDP with simultaneous synthesis of ATP from ADP + Pi. In three steps (4, 6, 10) in this cycle NAD+ is reduced to NADH + H+ and at step 8 (Figure 14.8) where FAD is reduced to FADH2.
The summary of link reaction and Krebs cycle in Mitochondria is
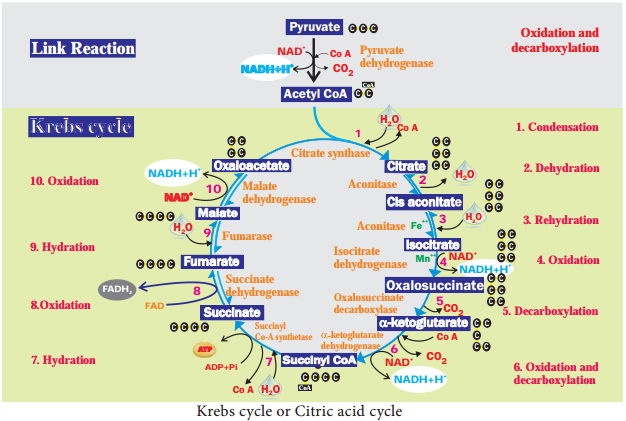
Two molecules of pyruvic acid formed at the end of glycolysis enter into the mitochondrial matrix. Therefore, Krebs cycle is repeated twice for every glucose molecule where two molecules of pyruvic acid produces six molecules of CO2, eight molecules of NADH + H+, two molecules of FADH2 and two molecules of ATP.
1. Significance of Krebs Cycle:
1. TCA cycle is to provide energy in the form of ATP for metabolism in plants.
2. It provides carbon skeleton or raw material for various anabolic processes.
3. Many intermediates of TCA cycle are further metabolised to produce amino acids, proteins and nucleic acids.
4. Succinyl CoA is raw material for formation of chlorophylls, cytochrome, phytochrome and other pyrrole substances.
5. α-ketoglutarate and oxaloacetate undergo reductive amination and produce amino acids.
6. It acts as metabolic sink which plays a central role in intermediary metabolism.
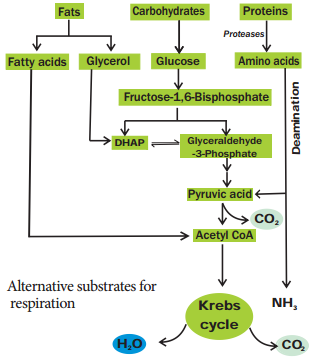
2. Amphibolic Nature
Krebs cycle is primarily a catabolic pathway, but it provides precursors for various biosynthetic pathways there by an anabolic pathway too. Hence, it is called amphibolic pathway. It serves as a pathway for oxidation of carbohydrates, fats and proteins.
When fats are respiratory substrate they are first broken down into glycerol and fatty acid. Glycerol is converted into DHAP and acetyl CoA. This acetyl CoA enter into the Krebs cycle.
When proteins are the respiratory substrate they are degraded into amino acids by proteases. The amino acids after deamination enter into the Krebs cycle through pyruvic acid or acetyl CoA and it depends upon the structure.
So respiratory intermediates form the link between synthesis as well as breakdown. The citric acid cycle is the final common pathway for oxidation of fuel molecules like amino acids, fatty acids and carbohydrates. Therefore, respiratory pathway is an amphibolic pathway (Figure 14.9).
Electron Transport Chain (ETC) (Terminal Oxidation)
During glycolysis, link reaction and Krebs cycle the respiratory substrates are oxidised at several steps and as a result many reduced coenzymes NADH + H+ and FADH2 are produced. These reduced coenzymes are transported to inner membrane of mitochondria and are converted back to their oxidised forms produce electrons and protons.
In mitochondria, the inner membrane is folded in the form of finger projections towards the matrix called cristae. In cristae many oxysomes (F1 particles) are present which have electron transport carriers. According to Peter Mitchell’s Chemiosmotic theory this electron transport is coupled to ATP synthesis. Electron and hydrogen(proton) transport takes place across four multiprotein complexes(I-IV). They are:-
1. Complex-I (NADH Dehydrogenase):
It contains a flavoprotein(FMN) and associated with non-heme iron Sulphur protein (Fe-S). This complex is responsible for passing electrons and protons from mitochondrial NADH (Internal) to Ubiquinone (UQ).
NADH + H+ + UQ ⇄ NAD+ + UQH2
In plants, an additional NADH dehydrogenase (External) complex is present on the outer surface of inner membrane of mitochondria which can oxidise cytosolic NADH + H+. Because mitochondrial inner membrane cannot allow NADH molecules directly into the matrix. Ubiquinone (UQ) or Coenzyme Quinone (CoQ) is a small, lipid soluble electron, proton carrier located within the inner membrane of mitochondria.
2. Complex-II (Succinic Dehydrogenase)
It contains FAD flavoprotein is associated with non-heme iron Sulphur (Fe-S) protein. This complex receives electrons and protons from succinate in Krebs cycle and is converted into fumarate and passes to ubiquinone. Succinate + UQ → Fumarate + UQH2
3. Complex-III (Cytochrome bc1 Complex)
This complex oxidises reduced ubiquinone (ubiquinol) and transfers the electrons through Cytochrome bc1 Complex (Iron Sulphur center bc1 complex) to cytochrome.
Cytochrome c is a small protein attached to the outer surface of inner membrane and act as a mobile carrier to transfer electrons between complex III to complex IV.
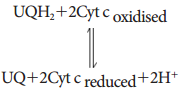
4. Complex IV (Cytochome Oxidase)
This complex contains two copper centers (A and B) and cytochromes a and a3. Complex IV is the terminal oxidase and brings about the reduction of 1/2 O2 to H2O. Two protons are needed to form a molecule of H2O (terminal oxidation).
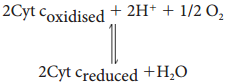
The transfer of electrons from reduced coenzyme NADH to oxygen via complexes I to IV is coupled to the synthesis of ATP from ADP and inorganic phosphate (Pi) which is called Oxidative phosphorylation. The F0F1. F1 converts ADP and Pi to ATP and is attached to the matrix side of the inner membrane. F0 is present in inner membrane and acts as a channel through which protons come into matrix.
Oxidation of one molecule of NADH + H+ gives rise to 3 molecules of ATP and oxidation of one molecule FADH2 produces 2 molecules of ATP within a mitochondrion. But cytoplasmic NADH + H+ yields only two ATPs through external NADH dehydrogenase.
Therefore, two reduced coenzyme (NADH + H+) molecules from glycolysis being extra mitochondrial will yield 2 × 2 = 4 ATP molecules instead of 6 ATPs (Figure 14.10). The Mechanism of mitochondrial ATP synthesis is based on Chemiosmotic hypothesis. According to this theory electron carriers present in the inner mitochondrial membrane allow for the transfer of protons (H+). For the production of single ATP, 3 protons (H+) are needed.
The terminal oxidation of external NADH bypasses the first phosphorylation site and hence only two ATP molecules are produced per external NADH oxidised through mitochondrial electron transport chain. However, in those animal tissues in which malate shuttle mechanism is present, the oxidation of external NADH will yield almost 3 ATP molecules.
Complete oxidation of a glucose molecule in aerobic respiration results in the net gain of 36 ATP molecules in plants as shown in table 14.2. Since huge amount of energy is generated in mitochondria in the form of ATP molecules they are called ‘power house of the cell’. In the case of aerobic prokaryotes due to lack of mitochondria each molecule of glucose produces 38 ATP molecules.
|
Stages |
CO2 | ATP | Reduced NAD+ | Reduced FAD |
Total ATP |
| Glycolysis | 0 | 2 | 2(2 × 2 = 4) | 0 | 6 |
| Link reaction | 2 | 0 | (2 × 3) = 6 | 0 | 6 |
| Krebs cycle | 4 | 2 | (6 × 3 = 18) | 2 (2 × 2 = 4) | 24 |
| Total | 6 | 4 ATPs | 28 ATPs | 4 ATPs | 36 ATPs |
Recent View
When the cost of transport of ATPs from matrix into the cytosol is considered, the number will be 2.5 ATPs for each NADH + H+ and 1.5 ATPs for each FADH2 oxidised during electron transport system. Therefore, in plant cells net yield of 30 ATP molecules for complete aerobic oxidation of one molecule of glucose. But in those animal cells (showing malate shuttle mechanism) net yield will be 32 ATP molecules.