Learninsta presents the core concepts of Biology with high-quality research papers and topical review articles.
Cell Division and its Difference Phases
Amitosis (Direct Cell Division)
Amitosis is also called direct or incipient cell division. Here there is no spindle formation and chromatin material does not condense. It consist of two steps: (Figure 7.2).
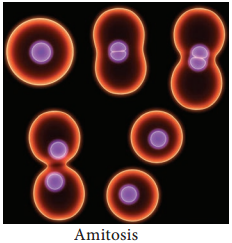
Karyokinesis:
- Involves division of nucleus.
- Nucleus develops a constriction at the center and becomes dumbell shaped.
- Constriction deepens and divides the nucleus into two.
Cytokinesis:
- Involves division of cytoplasm.
- Plasma membrane develops a constriction along nuclear constriction.
- It deepens centripetally and finally divides the cell into two cells.
Example:
Cells of mammalian cartilage, macronucleus of Paramecium and old degenerating cells of higher plants.
Drawbacks of Amitosis
- Causes unequal distribution of chromosomes.
- Can lead to abnormalities in metabolism and reproduction.
Mitosis
Mitosis occurs in shoot and root tips and other meristematic tissues of plants associated with growth. The number of chromosomes in the parent and the daughter (Progeny) cells remain the same so it is also called as equational division.
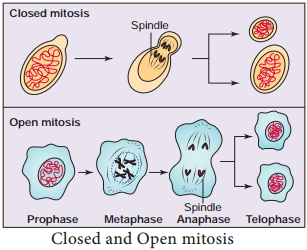
Closed and Open Mitosis
In closed mitosis, the nuclear envelope remains intact and chromosomes migrate to opposite poles of a spindle within the nucleus (Figure 7.3). Example: Many single celled eukaryotes including yeast and slime molds. In open mitosis, the nuclear envelope breaks down and then reforms around the 2 sets of separated chromosome. Example: Most plants and animals.
Mitosis is divided into four stages prophase, metaphase, anaphase and telophase (Figure 7.6).
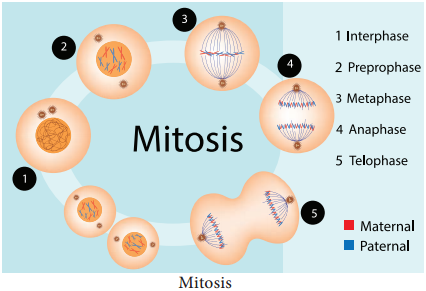
Prophase
Prophase is the longest phase in mitosis. Chromosomes become visible as long thin thread like structure, condenses to form compact mitotic chromosomes. In plant cells initiation of spindle fires takes place, nucleolus disappears. Nuclear envelope breaks down. Golgi apparatus and endoplasmic reticulum disappear.
In animal cell the centrioles extend a radial array of microtubules (Figure 7.4) and reach the poles of the cell. This arrangement of microtubules is called an aster. Plant cells do not form asters.
Metaphase
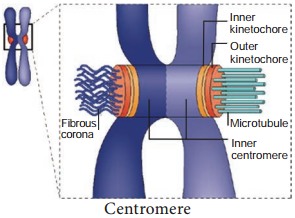
Chromosomes (two sister chromatids) are attached to the spindle fires by kinetochore of the centromere. The spindle fires are made up of tubulin. The alignment of chromosome into compact group at the equator of the cell is known as metaphase plate.
This is the stage where the chromosomal morphology can be easily studied. Kinetochore is a DNA-Protein complex present at the centromere where microtubules are attached. It is a trilaminar disc like plate.
Anaphase
Each chromosome splits simultaneously and two daughter chromatids begin to migrate towards two opposite poles of a cell. Each centromere splits longitudinally into two, freeing the two sister chromatids from each other. When sister chromatids separate the actual partitioning of the replicated genome is complete.
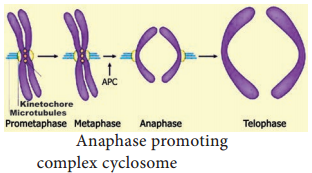
APC (Anaphase Promoting Complex) is a cluster of proteins that induces the breaking down of cohesion proteins which leads to the separation of chromatids during mitosis (Figure 7.5). This it helps in the transition of metaphase to anaphase.
Telophase
Two sets of daughter chromosomes reach opposite poles of the cell and mitotic spindle disappears. Division of genetic material is completed during karyokinesis, followed by cytokinesis (division of cytoplasm). Nucleolus and nuclear membranes reforms. Nuclear membrane form around each set of chromosomes. Now the chromosomes decondense.
In plants, phragmoplast are formed between the daughter cells. Cell plate is formed between the two daughter cells, reconstruction of cell wall takes place. Finally cells are separated by the distribution of organelles, macromolecules into two newly formed daughter cells.
Cytokinesis
Cytokinesis in Animal Cells It is a contractile process. The ring consists of a bundle of microfilaments assembled from actin and myosin. This firil generates a contractile force, that draws the ring inward forming a cleavage furrow in the cell. This it divides the cell into two.
Cytokinesis in Plant Cell
Division of the cytoplasm often starts during telophase. In plants, cell plate grows from centre towards lateral walls. Phragmoplast contains microtubules, actin filaments and vesicles from golgi apparatus and ER. Microtubule of the pharagmoplast move to the equator, fuse to form a new plasma membrane and the materials which are placed there becomes new cell wall.
The first stage of cell wall construction is a line dividing the newly forming cells called a cell plate. The cell plate eventually stretches right across the cell forming the middle lamella. Cellulose builds up on each side of the middle lamella to form the cell walls of two new plant cells.
Significance of Mitosis
Exact copy of the parent cell is produced by mitosis (genetically identical).
1. Genetic stability:
Daughter cells are genetically identical to parent cells.
2. Growth:
As multicellular organisms grow, the number of cells making up their tissue increases. The new cells must be identical to the existing ones.
3. Repair of Tissues:
Damaged cells must be replaced by identical new cells by mitosis.
4. Asexual Reproduction:
Asexual reproduction results in offspring that are identical to the parent. Example Yeast and Amoeba.
5. Flowering Plants:
In flowering plants, structure such as bulbs, corms, tubers, rhizomes and runners are produced by mitotic division. When they separate from the parent, they form a new individual.
The production of large numbers of offsprings in a short period of time, is possible only by mitosis. In genetic engineering and biotechnology, tissues are grown by mitosis (i.e. in tissue culture).
6. Regeneration:
Arms of star fish.
Meiosis
In Greek meioum means to reduce. Meiosis is unique because of synapsis, homologous recombination and reduction division. Meiosis takes place in the reproductive organs. It results in the formation of gametes with half the normal chromosome number.
Haploid sperms are made in testes; haploid eggs are made in ovaries of animals. In flowering plants meiosis occurs during microsporogenesis in anthers and megasporogenesis in ovule. In contrast to mitosis, meiosis produces cells that are not genetically identical. So meiosis has a key role in producing new genetic types which results in genetic variation.
Stages in Meiosis
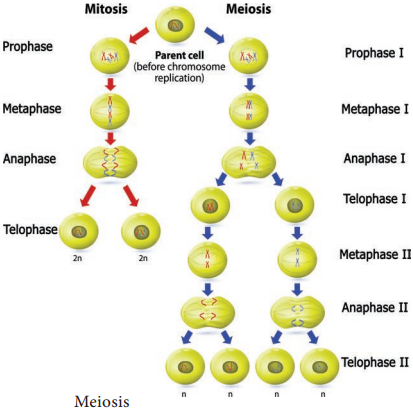
Meiosis can be studied under two divisions i.e., meiosis I and meiosis II. As with mitosis, the cell is said to be in interphase when it is not dividing.
Meiosis I:
Reduction Division
Prophase I:
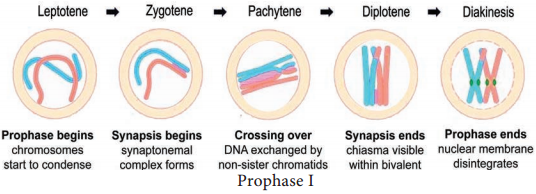
Prophase I is of longer duration and it is divided into 5 substages – Leptotene, Zygotene, Pachytene, Diplotene and Diakinesis (Figure 7.7).
Leptotene:
Chromosomes are visible under light microscope. Condensation of chromosomes takes place. Paired sister chromatids begin to condense.
Zygotene:
Pairing of homologous chromosomes takes place and it is known as synapsis. Chromosome synapsis is made by the formation of synaptonemal complex. The complex formed by the homologous chromosomes are called as bivalent (tetrads).
Pachytene:
At this stage bivalent chromosomes are clearly visible as tetrads. Bivalent of meiosis I consists of 4 chromatids and 2 centromeres. Synapsis is completed and recombination nodules appear at a site where crossing over takes place between non-sister chromatids of homologous chromosome. Recombination of homologous chromosomes is completed by the end of the stage but the chromosomes are linked at the sites of crossing over. This is mediated by the enzyme recombinase.
Diplotene:
Synaptonemal complex disassembled and dissolves. The homologous chromosomes remain attached at one or more points where crossing over has taken place. These points of attachment where ‘X’ shaped structures occur at the sites of crossing over is called Chiasmata.
Chiasmata are chromatin structures at sites where recombination has been taken place. They are specialised chromosomal structures that hold the homologous chromosomes together.
Sister chromatids remain closely associated whereas the homologous chromosomes tend to separate from each other but are held together by chiasmata. This substage may last for days or years depending on the sex and organism.
Diakinesis:
Terminalisation of chiasmata, homologous chromosomes become short and condensed. Nucleolus and nuclear envelope disappears. Spindle fires assemble.
Metaphase I
Spindle fires are attached to the centromeres of the two homologous chromosomes. Bivalent (pairs of homologous chromosomes) aligned at the equator of the cell known as metaphase plate. The random distribution of homologous chromosomes in a cell in Metaphase I is called independent assortment.
Anaphase I
Homologous chromosomes are separated from each other by shortening of spindle fiers. Each homologous chromosomes with its two chromatids and undivided centromere move towards the opposite poles of the cells. The actual reduction in the number of chromosomes takes place at this stage. Homologous chromosomes which move to the opposite poles are either paternal or maternal in origin. Sister chromatids remain attached with their centromeres.
Telophase I
Haploid set of chromosomes are present at each pole. The formation of two daughter cells, each with haploid number of chromosomes takes place. Nuclei reassembled. Nuclear envelope forms around the chromosome and the chromosomes becomes uncoiled. Nucleolus reappears.
In plants after karyokinesis, cytokinesis takes place by which two daughter cells are formed by the cell plate between 2 groups of chromosomes known as dyad of cells (haploid). The stage between the two meiotic divisions is called interkinesis which is short-lived.
Meiosis II:
Equational Division
This division is otherwise called mitotic meiosis because it resembles mitosis. Since it includes all the stages of mitotic divisions.
Prophase II
The chromosome with 2 chromatids becomes short, condensed, thick and becomes visible. New spindle develops at right angles to the cell axis. Nuclear membrane and nucleolus disappear.
Metaphase II
Chromosome arranged at the equatorial plane of the spindle. Microtubules of spindle gets attached to the centromere of sister chromatids.
Anaphase II
Sister chromatids separate. The daughter chromosomes move to the opposite poles due to shortening of spindle fires. Centromere of each chromosome split, allowing to move towards opposite poles of the cells holding the sister chromatids.
Telophase II
Four groups of chromosomes are organised into four haploid nuclei. The spindle disappears. Nuclear envelope, nucleolus reappear. After karyokinesis, cytokinesis follows and four haploid daughter cells are formed, called tetrads.
Signifiance of Meiosis
- This maintains a definite constant number of chromosomes in organisms.
- Crossing over takes place and exchange of genetic material leads to variations among species.
- These variations are the raw materials to evolution.
- Meiosis leads to genetic variability by partitioning different combinations of genes into gametes through independent assortment.
- Adaptation of organisms to various environmental stress.
Difference Between Mitosis in Plants and Animals
Plants | Animals |
| Centrioles are absent | Centrioles are present |
| Asters are not formed | Asters are formed |
| Cell division involves the formation of a cell plate | Cell division involves furrowing and cleavage of cytoplasm |
| Occurs mainly at meristem | Occurs in tissues throughout the body |
Mitosis | Meiosis |
| One division | Two divisions |
| Number of chromosome remain the same | Number of chromosomes is halved |
Homologous chromosomes line up
separately on the metaphase plate | Homologous chromosomes line up in pairs at the
metaphase plate |
| Homologous chromosome do not pair up | Homologous chromosome pairup to form bivalent |
Chiasmata do not form and crossing over
never occurs | Chiasmata form and crossingover occurs |
| Daughter cells are genetically identical | Daughter cells are genetically diffrent from parent cell |
| Two daughter cells are formed | Four daughter cells are formed |