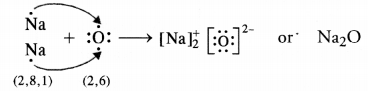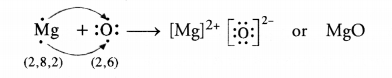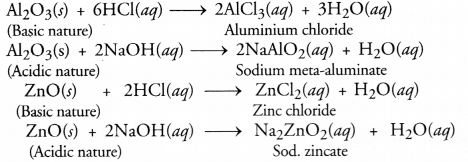NCERT Solutions for Class 10 Science Chapter 8 How do Organisms Reproduce
These Solutions are part of NCERT Solutions for Class 10 Science. Here we have given NCERT Solutions for Class 10 Science Chapter 8 How do Organisms Reproduce. Learn Insta provides you the Free PDF download of NCERT Solutions for Class 10 Science (Biology) Chapter 8 – How do Organisms Reproduce? solved by Expert Teachers as per NCERT (CBSE) Book guidelines. All Chapter 8 – How do Organisms Reproduce? Exercise Questions with Solutions to help you to revise complete Syllabus and Score More marks.
NCERT Questions
In Text Questions
Question 1.
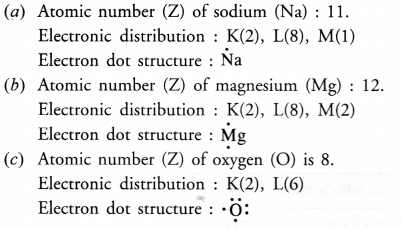
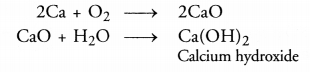
What is the importance of DNA copying in reproduction ? (CCE 2011, 2015)
Answer:
DNA carries hereditary information not only for controlling cellular functions but also all the structural and functional traits of organism. It is because of the latter that single celled zygote is able to form the whole multicellular organism. During reproduction there is formation of new cells which must carry the same amount and type of hereditary information as present in the parent cell. This is accomplished by DNA copying, which occurs prior to each cell division. DNA copying is not error proof. Errors give rise to variations.
More Resources
- NCERT Solutions for Class 10 Science
- NCERT Exemplar Solutions for Class 10 Science
- HOTS Questions for Class 10 Science
- Value Based Questions in Science for Class 10
- Previous Year Question Papers for CBSE Class 10 Science
Question 2.
Why are variations beneficial to the species but not necessarily for the individual ? (CCE 2011, 2012)
Answer:
Many of the variations are pre-adaptations which have no immediate benefit to the individuals. However, they remain in the population. Whenever, environment undergoes a drastic change, the pre-adaptations present in some members of the population allow the latter to survive, grow and regain its former size. Therefore, it is not necessary that variations are beneficial to individuals developing them but can prove useful to the species.
Question 3.
How does binary fission differ from multiple fission ?
Answer:
| Binary Fission | Multiple Fission |
| 1. Products. It gives rise to two individuals. | It forms several (more than two) individuals. |
| 2. Conditions. Binary fission occurs under favourable conditions. | Multiple fission occurs both under favourable and unfavourable conditions. |
| 3. Nucleus. Nucleus of the parent cell divides only once to form two daughters. | Nucleus of the parent undergoes repeated divisions to form a number of daughter nuclei. |
| 4. Cytoplasm. Cytoplasm undergoes cleavage after each nuclear division. | Cytoplasm does not divide after every nuclear division. |
| 5. Residue. No part of the parent body is left unused. | A part of the body, covering and residual cytoplasm, is left behind. |
| Examples. Amoeba, Paramecium. | Examples. Plasmodium, Amoeba (encysted). |
Question 4.
How will an organism be benefited if it reproduces through spores ? (CCE 2011, 2012)
Answer:
Sporulation or spore formation is a method of asexual reproduction where each individual produces a number of spores. On germination each spore forms a new individual, e.g., Rhizopus.
- All the daughters formed through spores are genetically similar.
- Spores are a means of dispersal. They help in spreading the organism far and wide.
- Spores can also function as a means of perennation or passage through unfavourable conditions.
Question 5.
Can you think of reasons why more complex organism cannot give rise to new individuals through regeneration.
Answer:
Regeneration is the ability of an organism to replace lost or injured parts so as to form the whole individual from an incomplete form or fragment by remodelling and growth of somatic cells through dedifferentiation, division, morphogenesis and redifferentiation. The ability for regenerative multiplication is present in simpler organisms because most of their cells can undergo dedifferentiation. However, it is limited to certain cells in complex organisms.
- The stem cells of complex organisms can form lost tissues and organs but not the complete individual as the highly differentiated tissues and organs do not allow this.
- In complex organisms regeneration is under neurohormonal control. Fragments do not have nervous or hormonal stimulus to grow into complete organisms.
Question 6.
Why is vegetative propagation practised for growing some types of organisms ?
Answer:
Vegetative propagation is practised in a number of horticulturally and economically important plants because it is advantageous.
Advantages:
- Seedless Plants. Vegetative propagation is the only known method of multiplication of seedless plants, g., Banana, Sugarcane, Pineapple, Jasmine, some varieties of Orange, Rose.
- Uniform Yield. Seeds and fruits are of uniform quality, size, taste and aroma.
- Genetic Uniformity. Vegetative propagation gives a genetically uniform population.
- Good Qualities. Good qualities of a variety can be maintained indefinitely.
- Survival Rate. Survival rate of the daughters is nearly 100% while in case of seed grown plants, it is 10%.
- Quicker Method. Vegetatively reproduced plants bear flowers and fruits earlier than the plants raised through seeds. Potato requires only three months for forming a new crop if raised from tubers. It takes 15 months if raised from seeds.
- Introduction in New Areas. In areas where seed germination fails to form mature plants, vegetative reproduction can help in establishing the plants.
Question 7.
Why is DNA copying an essential part of the process of reproduction ?
Answer:
Cell multiplication is essential for reproduction either as a means of multiplication in unicellular organisms or as a means of development of multicellular body from a single celled zygote. Cell multiplication cannot occur without DNA replication or DNA copying because each new cell must carry the full DNA complement.
Question 8.
How is the process of pollination different from fertilization ?
Answer:
| Pollination | Fertilization |
| 1. Definition: It is transfer of pollen grains from anther to the stigma of a flower. 2. Step: Pollination precedes fertilization. 3. Purpose: It carries the male gamete producing pollen grains to the female sex organ. 4. Process: Pollination is a physical process. 5. Occurrence: It occurs only in seed plants. | It is the fusion of male and female gametes. Fertilization occurs only after pollination when the pollen grain has germinated and male gametes are carried into ovule. It actually brings about fusion of gametes. Fertilization is a physico-chemical (biological) process. It occurs in both plants and animals of various types. |
Question 9.
What is the role of seminal vesicles and the prostate gland ? (CGE 2011, 2012)
Answer:
Seminal Vesicles: They secrete 60-70% of semen plasma that is alkaline and viscous having fructose (for nourishing the sperms), fibrinogen, proteins and prostaglandins. Prostaglandins cause movements in the genital tract of the female. Sperms are also activated by secretion of seminal vesicles.
Prostate Gland: It produces 20-30% of semen plasma. The secretion is alkaline and viscous. It has clotting enzyme and chemical essential for sperm activity.
Question 10.
What are the changes seen in girls at the time of puberty ?
Answer:
- Breast size begins to increase. There is darkening of skin of nipples below the tips of breasts.
- Menarche or beginning of menstruation.
- Broadening of pelvis,
- Deposition of fat in face, buttocks and thighs,
- Increased vasculature of skin and hence increased warmth of skin,
- Rounding of body ccthtours.
- High pitched voice,
- Slow growth of ovaries, fallopian tubes, uterus, vagina, enlargement of labia, etc.
Question 11.
How does the embryo get nourishment inside the mother’s body ? (CCE 2012)
Answer:
Embryo gets nourishment from mother’s body with the help of placenta through a cord called umbilical cord. Placenta contains many finger-like villi from the chorion covering of the embryo. They occur in contact with blood sinuses of the mother present in the endometrial lining of uterus. All nutrients (glucose, amino acids, vitamins, etc.) diffuse from mother’s blood into villi and from there to embryo through the umbilical cord.
Question 12.
If a woman is using a copper-T, will it help in protecting her from sexually transmitted diseases ?
Answer:
No, sexually transmitted diseases occur due to fluid to fluid contact that takes place in the vagina.
NCERT Chapter End Exercises
Question 1.
Asexual reproduction takes place through budding in
(A) Amoeba
(B) Yeast
(C) Plasmodium
(D) Leishmania.
Answer:
(B).
Question 2.
Which of the following is not a part of the female reproductive system in human beings.
(A) Ovary
(B) Uterus
(C) Vas deferens
(D) Fallopian tube.
Answer:
(C).
Question 3.
The anther contains
(A) Sepals
(B) Ovules
(C) Carpel
(D) Pollen grains.
Answer:
(D).
Question 4.
What are the advantages of sexual reproduction over asexual reproduction ?
Answer:
Asexual reproduction is monoparental, with no gametes, no meiosis and very little variations. Sexual reproduction is generally biparental involving fusion of gametes, meiosis and lot of variations.
Question 5.
What are the functions performed by testis in human beings ? (CCE 2011)
Answer:
- Formation of sperms from germinal cells found in seminiferous tubules.
- Secretion of hormone testosterone by Leydig cells. Testosterone induces secondary sexual characters at puberty. It helps in maintenance and functioning of secondary sex organs.
Question 6.
Why does menstruation occur ?
Answer:
Menstruation occurs in response to low level of estrogen and progesterone hormones which causes constriction of blood vessels in uterine wall, stoppage of nourishment to overgrown endometrium that sloughs off, passing out broken mucosal membrane, blood and mucus.
Question 7.
Draw a labelled diagram of L.S. flower.
Answer:
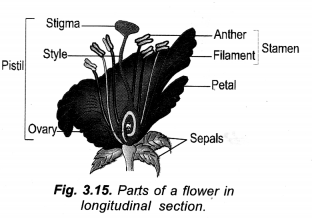
Question 8.
What are the different methods of contraception ?
Answer:
- Mechanical Barriers like condoms, cervical cap, diaphragm.
- Oral Contraceptives or oral pills like Mala D, Saheli
- Intrauterine Contraceptive Devices (IUCD) like loop, bow, Cu-T.
- Surgical Methods like vasectomy in males and tubectomy in females.
Question 9.
How are the modes for reproduction different in unicellular and multicellular organisms ?
Answer:
| Unicellular Organisms | Multicellular Organisms |
| 1. Reproductive Cell. The same cell which functions as the body of the organism also gets transformed into reproductive cell. | Specific cells take part in reproduction. |
| 2. Technique. Techniques of reproduction are simple. | Techniques of reproduction are commonly complex. |
| 3. Asexual Reproduction. It occurs through fission, budding and spore formation. | It occurs by several methods like fragmentation, regeneration, budding, spore formation, vegetative reproduction, etc. |
| 4. Sex Organs. No special sex cell or sex organ is present. | They are present. |
| 5. Sexual Reproduction. It occurs through isogamy to heterogamy. | It is commonly oogamous. |
Question 10.
How does reproduction help in providing stability to population of species ?
Answer:
- Replication of DNA.
- Growth and differentiation of cellular machinery.
- Cell division. It is mode of reproduction in single celled organisms,
- Development of special reproductive structures and formation of new individuals.
- Continued replication of DNA, growth and cell division, formation of tissues, organs, etc. and maturation into a multicellular organism.
Question 11.
What could be the reasons for adopting contraceptive methods ?
Answer:
- Enjoying a good reproductive health.
- Protecting from sexually transmitted diseases.
- Restricting the number of children.
- Spacing the birth of children so as to properly look after them, provide them proper education without depleting the resources of the family.
- Controlling population.
Selection Type Questions
Alternate Response Type Questions
(True/False (T/F), Right(√)/Wrong (x), Yes/No)
Question 1.
Basic event in reproduction is creation of DNA copy.
Question 2.
Plasmodium multiplies by binary fission.
Question 3.
Bryophyllum propagates through spore formation.
Question 4.
Copper-T is a contraceptive device used by women.
Question 5.
Hibiscus has unisexual flowers.
Question 6.
At the time of birth a girl baby has thousands of immature eggs.
Question 7.
Ovulation occurs in reproductively active females roughly in the middle of menstrual cycle.
Question 8.
Sperms mature at a temperature higher than that of human body.
Matching Type Questions
Question 9.
Match the articles given in columns A and B (single matching)

Question 10.
Match the contents of columns I, II and III (double matching) :

Question 11.
List the type of reproduction (A-asexual, V-vegetative, S-sexual) in the following organisms (Key or check list items)
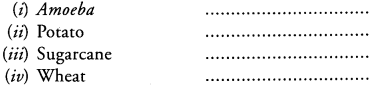
Question 12.
Match each stimulus with appropriate response :

Fill In the Blanks
Question 13. ………………….. help in survival of the species in changing environment.
Question 14. ………………….. is common method of multiplication of Yeast and Hydra.
Question 15. Reproductive tissues begin to …………………….. when rate of general body growth begins to ………………… .
Question 16. Fallopian tubes are cut and ligated in ………………… .
Question 17. Bartholin’s glands are components of ……………………. reproductive system.
Answers:
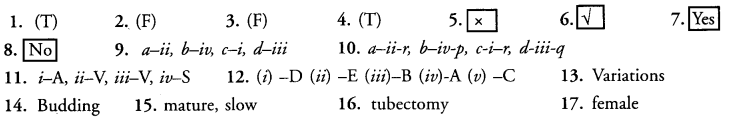
NCERT Solutions for Class 10 Science Chapter 8 – How do Organisms Reproduce?
Hope given NCERT Solutions for Class 10 Science Chapter 8 are helpful to complete your science homework.
If you have any doubts, please comment below. Learn Insta try to provide online science tutoring for you.