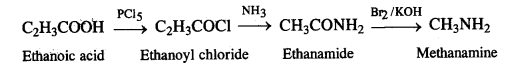
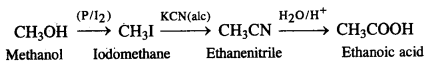
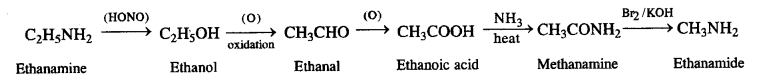
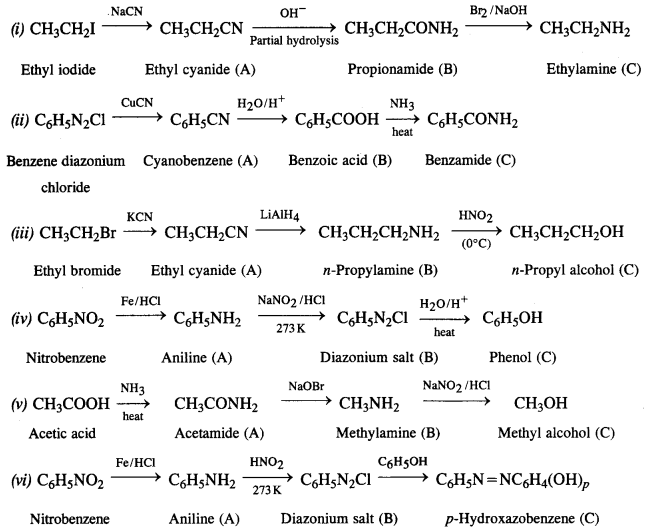
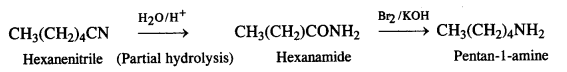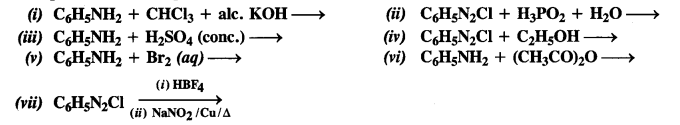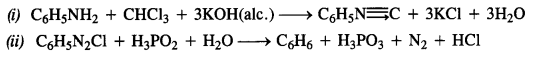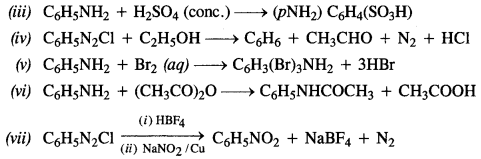NCERT Solutions for Class 7 English Honeycomb Chapter 2 A Gift of Chappals are part of NCERT Solutions for Class 7 English. Here we have given NCERT Solutions for Class 7 English Honeycomb Chapter 2 A Gift of Chappals.
| Board | CBSE |
| Textbook | NCERT |
| Class | Class 7 |
| Subject | English Honeycomb |
| Chapter | Chapter 2 |
| Chapter Name | A Gift of Chappals |
| Number of Questions Solved | 38 |
| Category | NCERT Solutions |
NCERT Solutions for Class 7 English Honeycomb Chapter 2 A Gift of Chappals
IMPORTANT PASSAGES FOR COMPREHENSION
Read the following extracts and answer the questions that follow by choosing the correct option :
[I]
Question 1.
“People are always telling us to be kind to animals, but when we are, they scream. Ooh don’t bring that dirty creature here !” said Ravi. “Do you know how hard it is just to get a little milk from the kitchen ? (Page 19)
Multiple Choice Questions
Question 1.
Identify ‘people’ and ‘we’
(a) colony members and the family
(b) family members and the group
(c) elders and children
(d) environmentalists and children
Answer.
(c) elders and children
Question 2.
Meena shares with Mridu
(a) the biryani cooked by Rukku Mani
(b) the secret about the cat in the backyard
(c) the chocolate Ravi brought
(d) the advice given by the beggar that
Answer.
(b) the secret about the cat in the backyard
Question 3.
Ravi poured the milk for the kitten
(a) the kitchen
(b) the fridge
(c) the market
(d) the dairy
Answer.
(a) the kitchen
Question 2.
“She’ll never learn a thing. The train whizzing on and on, while Lalli’s all the time-derailing ! Going completely off track !” (Page 21)
Question 1.
Who is the speaker of the above extract ?
Answer.
The speaker of the above extract is Ravi.
Question 2.
What is the music master trying to do ?
Answer.
The music master is trying to teach Lalli music.
Question 3.
Is he successful in his effort ?
Answer.
The music master is unsuccessful. Lalli is not able to leam.
[II]
Question 3.
“He has been coming here every day for the past week and it’s time he found another house to beg from !” Paati explained to Tapi. (Page 24)
Multiple Choice Questions
Question 1.
Paati explained to Tapi that the beggar
(a) was very notorious
(b) should beg from some other house
(c) should find some other person
(d) never listened to her
Answer.
(b) should beg from some other house
Question 2.
The beggar raised his voice to
(a) beg for money
(b) beg for alms
(c) beg for food and rest
(d) beg for rest
Answer.
(c) beg for food and rest
Question 3.
Rukku Manni told Ravi to tell the beggar
(a) not to come again
(b) to take food
(c) to rest under the tree
(d) to find food elsewhere
Answer.
(a) not to come again
Question 4.
In two minutes he’ll be fiying his feet on that road. (Page 25)
Question 1.
Who is the speaker of the above line ?
Answer.
The speaker of the above line is Ravi.
Question 2.
Who is ‘he’ in the above line ?
Answer.
‘He’ refers to the beggar.
Question 3.
Why should ‘he’ be frying his feet on the road ?
Answer.
The beggar has no shoes or chappals for his feet. As it is a hot day, Ravi thinks that the poor beggar will be frying his (bare) feet on the road.
Question 5.
“These should fit you, Sir. Please put these on. I gun so sorry. My son has been naughty.” (Page 27)
Multiple Choice Questions
Question 1.
Rukku Manni offered chappals that were
(a) old and worn out
(b) new
(c) big in size
(d) small in size
Answer.
(b) new
Question 2.
On seeing the chappals, the music-master
(a) was too happy
(b) grew angiy
(c) picked them to keep in his bag
(d) sat down to wear them
Answer.
(a) was too happy
Question 3.
Ravi’s chappals were in question as
(a) he would not have given his chappals if they were a perfect fit
(b) he would have given his own chappals if they were a perfect fit
(c) his chappals fitted the beggar’s feet
(d) his chappals did not fit the beggar’s feet
Answer.
(b) he would have given his own chappals if they were a perfect fit
TEXTUAL QUESTIONS
Comprehension Check (Page 22)
Question 1.
What is the secret that Meena shares with Mridu in the backyard ?
Answer.
Meena shares with Mridu the secret about the cat in the backyard. The secret was that they had a kitten hidden behind the bitter berry bush.
Question 2.
How does Ravi get milk for the kitten ?
Answer.
Ravi said that he was hungry. So he got some milk from the kitchen. Ravi poured milk into the coconut shell. He then washed the tumbler and put it back. Thus Ravi got milk for the kitten.
Question 3.
Who does he say the kitten’s ancestors are ? Do you believe him ?
Answer.
He says that the kitten’s ancestors are the Pallavas. No, I don’t believe him.
Question 4.
Ravi has a lot to say about M.P. Poonai This shows that
- he is merely trying to impress Mridu.
- his knowledge of history is sound.
- he has a rich imagination.
- he is an intelligent child.
Which of these statements do you agree I disagree to ?
Answer.
We agree with the statements (1) he is merely trying to impress Mridu and (3) he has a rich imagination.
Question 5.
What was the noise that startled Mridu and frightened Mahendran ?
Answer.
A ‘kreeching’ sound startled Mridu and frightened Mahendran.
Comprehension Check (Page 28)
Question 1.
The music master is making lovely music. Read aloud the sentence in the text that expresses this idea.
Answer.
The music master’s notes seemed to float up and settle perfectly into the visible tracks of the melody.
Question 2.
Had the beggar come to Rukku Manni’s house for the first time ? Give reasons for your answer.
Answer.
No. The beggar had not come to Rukku Manni’s house for the first time. The beggar himself says that he had been coming there regularly for a week.
Question 3.
“A sharp V-shaped line hadformed between her eyebrows. ” What does it suggest to you about Rukku Manni’s mood ?
Answer.
A sharp V-shaped line between her eyebrows suggests that Rukku Manni was losing patience. She was getting angry.
Working with the Text
Question 1.
Complete the following sentences :
(i) Ravi compares Lalli’s playing the violin to ………………………………………
(ii) Trying to hide beneath the tray of chillies. Mahendran ……………………
(iii) The teacher played a few notes on his violin, and Lalli ……………………
(iv) The beggar said that the kind ladies of the household …………………….
(v) After the lesson was over, the music teacher asked Lalli if ………………..
Answers.
- a railway train which is all the time derailing, going completely off the track.
- tipped a few chillies over himself.
- stumbled behind him on her violin without much success.
- had helped him with food for a whole week.
- she had seen his chappals.
Question 2.
Describe the music teacher, as seen from the window.
Answer.
The music teacher sat in front of Lalli with most of his back to the window. He was bony. His head was bald. A fringe of oiled black hair fell around his ears. He had an old fashioned tuft. He had a thick neck. Round it he wore a gold chain. He had a diamond on his hand. He had a scrawny big toe.
He was playing on the violin with his hands. He beat time on the floor with his toe.
Question 3.
(i) What makes Mridu conclude that the beggar has no money to buy chappals ? (Imp.)
(ii) What does she suggest to show her concern ?
Answers.
- The beggar showed the children his bare feet. There were large blisters on them. So Mridu concluded that the beggar had no money to buy chappals.
- To show her concern for the beggar she suggests that an old pair of chappals should be given to him.
Question 4.
“Have you children…” she began, and then, seeing they were curiously quiet, went on more slowly, “seen anyone lurking around the verandah ?” (Imp.)
(i) What do you think Rukku Manni really wanted to ask ?
(ii) Why did she change her question ?
(iii) What did she think had happened ?
Answers.
- Rukku Manni really wanted to ask if the children had hidden the chappals.
- Seeing the children curiously quiet, she felt something more serious had happened.
So she changed her question. - She thought that the chappals had gone for good.
Question 5.
On getting Gopu Mama’s chappals, the music teacher tried not to look too happy. Why ?
Answer.
The music master did not like to show his greed. So, although his eyes lit up, he tried not to look too happy.
Question 6.
On getting a gift of chappals, the beggar vanished in a minute. Why was he in such a hurry to leave ?
Answer.
The beggar had realised that the children had given him chappals of their own. They had not sought the permission of the elders. He feared that the elders could take it back. So he vanished in a minute.
Question 7.
Walking towards the kitchen with Mridu and Meena, Rukku Manni began to laugh. What made her laugh ? (Imp.)
Answer.
It was the mental picture of Gopu Mama which made Rukku Manni laugh. She knew he would feel very uncomfortable. On coming home, it was his habit to throw off his shoes and get into chappals as soon as possible.
Working with Language
Question 1.
Read the following sentences :
(a) If she knows we have a cat, Paati will leave the’ house. ‘
(b) She won’t be so upset if she knows about the poor beggar with sores on his feet.
(c) If the chappals do fit, will you really not mind ?
Notice that each sentence consists of two parts. The first part begins with ‘if. It is known as if-clause.
Rewrite each of the following pairs of sentences as a single sentence. Use ‘if at the beginning of the sentence.
(a) Walk fast. You’ll catch the bus.
If you walk fast, you’ll catch the bus.
(b) Don’t spit on the road. You’ll be fined.
If you spit on the road, you’ll be fined.
(i) Don’t tire yourself now. You won’t be able to work in the evening.
(ii) Study regularly. You’ll do well in the examination.
(iii) Work hard. You’ll pass the examination in the first division.
(iv) Be polite to people. They’ll also be polite to you.
(v) Don’t tease the dog. It’ll bite you.
Answers.
- If you tire yourself now, you won’t be able to work in the evening.
- If you study regularly, you’ll do well in the examination.
- If you work hard, you’ll pass the examination in the first division.
- If you are polite to people, they’ll also be polite to you.
- If you tease the dog, it’ll bite you.
Question 2.
Fill in the blanks in the following paragraph :
Today is Sunday. I’m wondering whether I should stay at home or go out. If I ……….. (go) out, I ……… (miss) the lovely Sunday lunch at home. If I …….. (stay) for lunch, I ……… (miss) the Sunday film showing at Archana Theatre. I think I’ll go out and see the film, only to avoid getting too fat.
Answer.
Today is Sunday. I’m wondering whether I should stay at home or go out. If I go out, I’ll miss the lovely Sunday lunch at home. If I stay for lunch, I’ll miss the Sunday film showing at Archana Theatre. I think I’ll go out and see the film, only to avoid getting too fat.
Question 3.
Complete each sentence below by appropriately using any one of the following :
if you want to/if you don’t want to/if you want him to
(i) Don’t go to the theatre ……………
(ii) He’ll post your letter ………………
(iii) Please use my pen ………………
(iv) He’ll lend you his umbrella ………..
(v) My neighbour, Ramesh, will take you to the doctor …………
(vi) Don’t eat it …………………
Answers.
- if you don’t want to.
- if you want him to.
- if you want to.
- if you want him to.
- if you want him to.
- if you don’t want to.
Speaking and Writing
Question 1.
Discuss in small groups
• If you want to give away something of your own to the needy, would it be better to ask your elders first ?
Answers.
A : If there is something which is our own, we needn’t seek permission to use it.
B : I don’t agree. We do not earn anything. What we have is given to us by our parents. So it is necessary to seek their permission to give it to someone else.
C : I am afraid, both of you are running to extremes. We have to take into consideration the value of the thing as well. For example, if we have two pencils, we may give one to a friend. However, we cannot do the same with a pair of shoes or a suit of clothes. We must seek the permission of our elders before offering it to someone. After all, it is they who will be burdened with extra expenditure.
• Is there someone of your age in the family who is very talkative ? Do you find her/him interesting and impressive or otherwise ? Share your ideas with oth¬ers in the group.
Answers.
A : I have a cousin. She is very talkative. Since we live in the same house, I have to tolerate her somehow. However, she causes much annoyance. I find it difficult to concentrate on my books.
B : I too have a talkative cousin. However, I love to hear her. Her knowledge is so vast and her voice so sweet. Whenever I am tired or bored with study, I go to her. Her conversation gives me a lot of joy. I feel refreshed and am ready to work again.
C : My sister is very talkative. However, I tell her not to talk much these days. These are examination days and I want to devote much time to study. Thankfully, she has accepted my request.
• Has Rukku Manni done exactly the same as children? In your opinion, then, is it right for one party to blame the other ?
Answers.
A : Yes. Rukku Manni did only what Ravi had done. So it was not at all right for her to scold Ravi.
B : I don’t agree. Rukku Manni was forced to do what she did. The music teacher’s chappals had gone. How to compensate him ? The only option left to her was to give Gopu Mama’s chappals to him. So we cannot equate the two acts. She was quite justified in blaming and scolding the children.
C : The chidren had certainly done something wrong. It is not correct and Rukku Manni’s act can’t be equated with theirs. However, I think, such children need a more sympathetic handling. They had taken pity on a beggar. They must be taught how far they can allow their sympathy to go. They should not have touched music teacher’s chappals.
Question 2.
Read the following :
• A group of children in your class are going to live in a hostel.
• They have been asked to choose a person in the group to share a room with.
• They are asking each other questions to decide who they would like to share a room with.
Ask one another questions about likes/dislikes/preferences/hobbies/personal characteristics. Use the following questions and sentence openings.
(i) What do you enjoy doing after school ?
I enjoy…
(ii) What do you like in general ?
I like…
(iii) Do you play any game ?
I don’t like…
(iv) Would you mind if I listened to music after dinner ?
I wouldn’t…
(v) Will it be all right if I… ?
It’s fine with me…
(vi) Is there anything you dislike, particularly ?
Well, I can’t share…
(vii) Do you like to attend parties ?
Oh, I…
(viii) Would you say you are… ?
I think…
Answer.
Please attempt yourself.
We hope the NCERT Solutions for Class 7 English Honeycomb Chapter 2 A Gift of Chappals help you. If you have any query regarding NCERT Solutions for Class 7 English Honeycomb Chapter 2 A Gift of Chappals, drop a comment below and we will get back to you at the earliest.