Find free online Chemistry Topics covering a broad range of concepts from research institutes around the world.
Alkali Earth Metals
Group 2 in the modern periodic table contains the elements beryllium, magnesium, calcium, strontium, barium and radium. These elements with the exception of beryllium are commonly known as the alkaline earth metals because their oxides and hydroxides are alkaline in nature and these metal oxides are found in the earth’s crust.

General Characteristics of Alkaline Earth Metals
Physical State
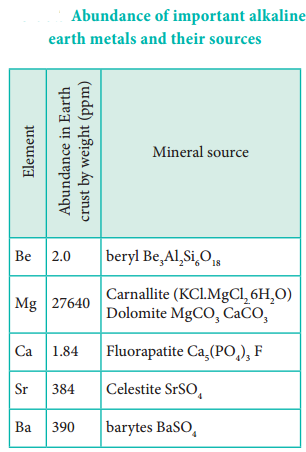
Beryllium is rare and radium is the rarest of all comprising only 10 % of igneous rocks. Magnesium and calcium are very common in the earth’s crust, with calcium the fifth-most-abundant element, and magnesium the eighth. Magnesium and calcium are found in many rocks and minerals: magnesium in carnallite, magnesite, dolomite and calcium in chalk, limestone, gypsum.
Most strontium is found in the minerals celestite and strontianite. Barium is slightly less common, much of it in the mineral barite. Radium, being a decay product of uranium, is found in all uranium-bearing ores.
Electronic Configuration
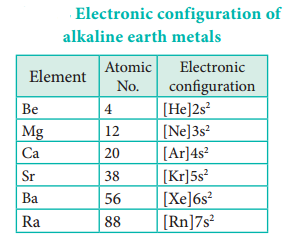
These elements have two electrons in the valence shell of their atoms, preceded by the noble gas configuration. Their general electronic configuration is written as [Noble gas]ns2 where ‘n’ represents the valence shell.
Atomic and Ionic Radii
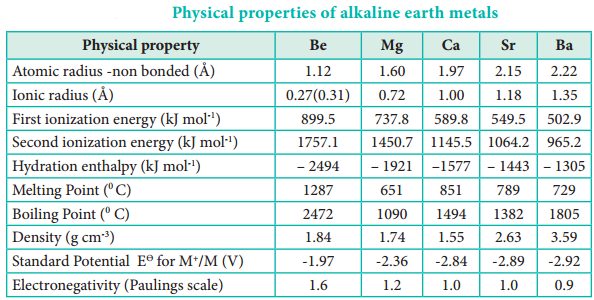
The atomic and ionic radii of alkaline earth metals are smaller than the corresponding members of the alkali metals. This is due to the fact the Group 2 elements having a higher nuclear charge that allows electrons to be attracted more strongly towards the nucleus. On moving down the group, the radii increases due to gradual increase in the number of the shells and the screening effect.
Common Oxidation State
The group 2 elements have two electrons in their valence shell and by losing these electrons, they acquire the stable noble gas configuration. So these elements exhibit +2 oxidation state in their compounds.
Ionisation Enthalpy
Due to a fairly large size of the atoms, alkaline earth metals have low ionisation enthalpies when compared to ‘p’ block elements. Down the group the ionisation enthalpy decreases as atomic size increases. This is due to the addition of new shells as well as increase in the magnitude of the screening effect of inner shell electrons. Members of group 2 have higher ionization enthalpy values than group 1 because of their smaller size, with electrons being more attracted towards the nucleus of the atoms. Correspondingly they are less electropositive than alkali metals.
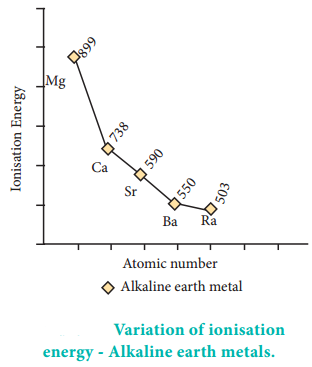
Although IE1 values of alkaline earth metals are higher than that of alkali metals, the IE2 values of alkaline
earth metals are much smaller than those of alkali metals. This occurs because in alkali metals the second electron is to be removed from a cation, which has already acquired a noble gas configuration. In the case of alkaline earth metals, the second electron is to be removed from a monovalent cation, which still has one electron in the outermost shell. Thus, the second electron can be removed more easily in the case of group 2 elements than in group 1 elements.
Hydration Enthalpies
Compounds of alkaline earth metals are more extensively hydrated than those of alkali metals, because the hydration enthalpies of alkaline earth metal ions are larger than those of alkali metal ions.
Like alkali metal ions, the hydration enthalpies of alkaline earth metal ions also decrease with increase in ionic size down the group.
Be > Mg > Ca > Sr > Ba
e.g., Magnesium chloride and calcium chloride exist as their hydrated crystals MgCl2.6H2O and CaCl2.6H2O
respectively. whereas NaCl and KCl do not form such hydrates.
Electronegativity
In alkaline earth metals the electronegativity values decrease as we go down the group as seen in the alkali metals.
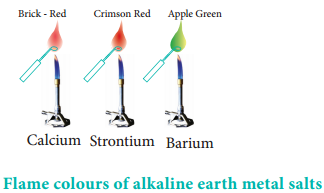
Flame Colour and the Spectra:
When the alkaline earth metal salts moistened with concentrated hydrochloric acid are heated on a platinum wire in a flame, they show characteristic coloured flame as shown below.
Table 5.10 Flame Color and Wavelength
|
Element |
Colour |
Wavelength (nm) |
| Calcium | Brick – Red | 622 |
| Strontium | Crimson – Red | 689 |
| Barium | Apple Green | 554 |
The heat in the flame excites the valence electron to a higher energy level. when it drops back to its actual energy level, the excess energy is emitted as light, whose wavelength is in the visible region as shown in the above table.
Distinctive Behaviour of Beryllium
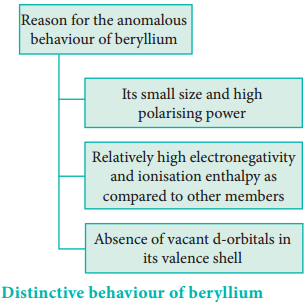
The anomalous properties of beryllium is mainly due to its small size, high electronegativity, high ionisation energy and high polarising power compared to the other elements in the block. The anomalous properties of beryllium compared to other elements of the group are mentioned in Table 5.11
|
Beryllium |
Other elements of the family |
| 1. Forms covalent compounds | 1. Form ionic compounds |
| 2. High melting and boiling point | 2. Low melting and boiling point |
| 3. Does not react with water even at elevated temperature | 3. React with water |
| 4. Does not combine directly with hydrogen | 4. Combine directly with hydrogen |
| 5. Does not combine directly with halogens. Halides are covalent. | 5. Combine directly with halogens Halides are electrovalent. |
| 6. Hydroxide and oxides of beryllium are amphoteric in nature |
6. Basic in nature. |
| 7. It is not readily attacked by acids because of the presence of an oxide fim | 7. Readily attacked by acids |
| 8. Beryllium carbide evolves methane with water. |
8. Evolve acetylene with water. |
| 9. Salts of Be are extensively hydrolysed | 9. Hydrolysed |
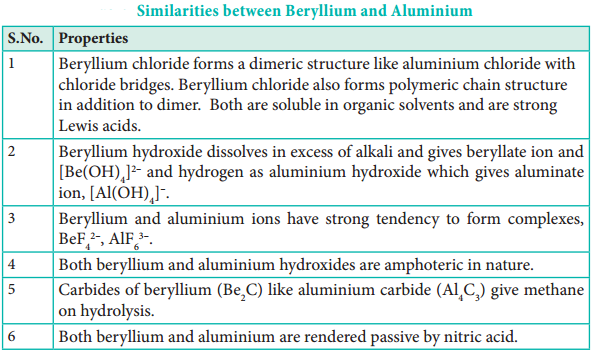
Diagonal Relationship:
As observed in alkali metals, beryllium (the first member of group 2) shows a diagonal relationship with aluminium. In this case, the size of these ions (rBe2+ = 0.45 Å and rAl3+ = 0.54 Å) is not as close. However, their charge per unit area is closer (Be2+ = 2.36 and Al3+ = 2.50). They also have same electronegativity values (Be = 1.5; Al = 1.5).
Table 5.12 Similarities Between Beryllium and Aluminium
Chemical Properties of Alkaline Earth Metals
The alkaline earth metals are less reactive than the alkali metals. The reactivity of these elements increases on going down the group.
Reactivity Towards the Halogens:
All the alkaline earth metals combine with halogen at elevated temperatures to form their halides.
M + X2 → MX2
(M = Be, Mg, Ca, Sr, Ba, Ra, X = F, Cl, Br, l)
Thermal decomposition of (NH4)2BeF4 is the best route for the preparation of BeF2. BeCl2 is conveniently made from the oxide.
![]()
Reactivity Towards Hydrogen:
All the elements except beryllium, combine with hydrogen on heating to form their hydrides with general formula MH2. BeH2 can be prepared by the reaction of BeCl2 with LiAlH4.
2BeCl2 + LiAlH4 → 2BeH2 + LiCl + AlCl3
Uses of Alkaline Earth Metals
Uses of Beryllium
- Because of its low atomic number and very low absorption for X-rays, it is used as radiation windows for X-ray tubes and X-ray detectors.
- The sample holder in X-ray emission studies usually made of beryllium
- Since beryllium is transparent to energetic particles, it is used to build the ‘beam pipe’ in accelerators.
- Because of its low density and diamagnetic nature, it is used in various detectors.
Uses of Magnesium
- Removal of sulphur from iron and steel.
- Used as photoengrave plates in printing industry.
- Magnesium alloys are used in aeroplane and missile construction.
- Mg ribbon is used in synthesis of Grignard reagent in organic synthesis.
- It alloys with aluminium to improve its mechanical, fabrication and welding property.
- As a desiccant.
- As sacrificial anode in controlling galvanic corrosion.
Uses of Calcium
- As a reducing agent in the metallurgy of uranium, zirconium and thorium.
- As a deoxidiser, desulphuriser or decarboniser for various ferrous and non-ferrous alloys.
- In making cement and mortar to be used in construction.
- As a getter in vacuum tubes.
- In dehydrating oils
- In fertilisers, concrete and plaster of paris.
Uses of Strontium
- 90Sr is used in cancer therapy.
- 87Sr/86Sr ratios are commonly used in marine investigations as well as in teeth, tracking animal migrations or in criminal forensics.
- Dating of rocks.
- As a radioactive tracer in determining the source of ancient archaeological materials such as timbers and coins.
Uses of Barium
- Used in metallurgy, its compounds are used in pyrotechnics, petroleum mining and radiology.
- Deoxidiser in copper refining.
- Its alloys with nickel readily emits electrons hence used in electron tubes and in spark plug electrodes.
- As a scavenger to remove last traces of oxygen and other gases in television and other electronic tubes.
- An isotope of barium 133Ba, used as a source in the calibration of gamma ray detectors in nuclear chemistry.
Uses of Radium
Used in self-luminous paints for watches, nuclear panels, aircraft switches, clocks and instrument dials.