In this page, we are providing Acids, Bases and Salts Class 7 Extra Questions and Answers Science Chapter 5 pdf download. NCERT Extra Questions for Class 7 Science Chapter 5 Acids, Bases and Salts with Answers will help to score more marks in your CBSE Board Exams.
Class 7 Science Chapter 5 Extra Questions and Answers Acids, Bases and Salts
Extra Questions for Class 7 Science Chapter 5 Acids, Bases and Salts with Answers Solutions
Acids, Bases and Salts Class 7 Extra Questions Very Short Answer Type
Question 1.
Give two examples of acidic substances.
Answer:
Lemon juice and vinegar.
Question 2.
Give two examples of basic substances.
Answer:
Lime water and baking soda.
Question 3.
What is the colour of litmus in distilled water?
Answer:
Mauve (purple) colour.
Question 4.
Name some substances in which tartaric acid is found?
Answer:
Tamarind, grapes, unripe mangoes, etc.
Question 5.
Which base is found in window cleaner?
Answer:
Ammonium hydroxide.
Question 6.
Give an example of an antacid.
Answer:
Milk of magnesia.
Question 7.
What makes the soil acidic?
Answer:
Excessive use of chemical fertilisers.
Question 8.
Which acid is injected into our skin when an ant bites?
Answer:
Formic acid.
Question 9.
What helps us to digest food?
Answer:
Hydrochloric acid helps us to digest food.
Question 10.
Name two types of litmus papers.
Answer:
Red litmus paper and blue litmus paper.
Question 11.
What colour does phenolphthalein give when the solution is basic?
Answer:
Pink
Question 12.
What neutralises the basic nature of the soil?
Answer:
Acids released by organic matter neutralises the basic nature of the soil.
Question 13.
When organic matter is added to the soil?
Answer:
If the soil is basic.
Question 14.
What substance is used to treat the soil when it is too acidic?
Answer:
Bases like quick lime or slaked lime.
Question 15.
What kills fish and other organism present in the water bodies?
Answer:
Releasing of factory wastes containing acids or bases.
Question 16.
What is nature of salt?
Answer:
It may be acidic, basic or neutral in nature.
Question 17.
What happens when an acidic solution is mixed with a basic solution?
Answer:
Both solution neutralises each other with the formation of salt, water and evolution of heat.
Question 18.
What is neutralisation?
Answer:
The reaction between an acid and a base is called neutralisation.
Question 19.
Name two natural indicator.
Answer:
- Litmus
- Turmeric Question
Question 20.
What is a neutral substance?
Answer:
The substance of solution which does not show any effect on litmus paper is called a neutral substance.
Question 21.
What is an acid?
Answer:
The substance having sour taste is called an acid.
Question 22.
What is a base?
Answer:
The substance having bitter taste and feels soapy on touching is called a base.
Acids, Bases and Salts Class 7 Extra Questions Short Answer Type
Question 1.
Explain the terms acids and acidic.
Answer:
The substances that taste sour are called acids, such as curd, lemon juice, orange juice, vinegar, etc. The chemical nature of these substances are acidic.
Question 2.
What are bases? What is their nature?
Answer:
The substances that taste bitter and feel soapy on touching are called bases; e.g., lime water, baking soda, washing soda, etc. The nature of these substances are basic.
Question 3.
What are indicators?
Answer:
Indicators are special type of substances that are used to test whether a substance is acidic or basic. They change their colour when added to a solution containing an acidic or a basic substance. For example, turmeric, China rose petals, litmus, etc., are naturally occurring indicators.
Question 4.
What is litmus?
Answer:
Litmus is the most commonly used natural indicator. It is extracted from lichens. It has mauve (purple) colour in distilled water when added to an acidic solution, it turns red and when added to a basic solution, it turns blue. It may be in the form of a solution or in the form of strips of paper called litmus paper, which are generally red and blue.
Question 5.
What is neutralisation? Give an example of a neutralisation reaction.
Answer:
The reaction between an acid and a base is called neutralisation. In this process, salt and water are formed with the evolution of heat.
Acid + Base ➝ Salt + Water + (heat is evolved)
Here is a reaction showing neutralisation.
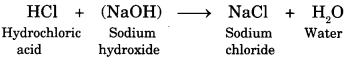
Question 6.
Why do you rub baking soda or calamine solution when an ant bites us?
Answer:
When an ant bites, it inject formic acid into our skin, which is painful and may be very harmful sometimes. The effect of the acid can be neutralised by rubbing moist baking soda, i.e., sodium hydrogen carbonate or calamine solution containing zinc carbonate.
Question 7.
How are the factory wastes neutralised?
Answer:
Many factory waste contain acids which kill fish and other organisms, if allowed to flow into the water bodies untreated. These wastes can be neutralised by adding basic substances to them.
Question 8.
What is salt?
Answer:
Salt is a substance obtained from the neutralisation reaction of an acid and a base.
Acid + Base ➝ Salt + Water + Heat
Salt may be acidic, basic or neutral depending on whether the acids or bases used to make salts are weak or strong.
Question 9.
What is meant by acid rain?
Answer:
The rain containing excess of acids is called acid rain. The rain becomes acidic because carbon dioxide, sulphur dioxide and nitrogen dioxide, which are released into the air as pollutants, dissolve in rain water to form carbonic acid, sulphuric acid and nitric acid respectively. Acid rain can cause damage to buildings, historical monuments, plants and animals.
Acids, Bases and Salts Class 7 Extra Questions Long Answer Type
Question 1.
Explain the process of neutralisation with the help of an activity.
Answer:
When an acidic solution is mixed with a basic solution, both the solutions neutralise the effect of each other. The resulting solution is neither acidic nor basic. We can show the process of neutralisation with the help of an activity.
Fill one-fourth of a test tube with dilute hydrochloric acid. Note down its colour and also the colour of . phenolphthalein solution. Add 2-3 drops of the indicator to the acid. Shake the test tube gently. We observe that solution remains colourless. Add sodium hydroxide solution in the test tube drop by drop with continuous stirring till the pink colour just appears. Appearance of pink colour indicates that the neutralisation reaction has completed.
Question 2.
What are the applications of neutralisation reaction in everyday life?
Answer:
Neutralisation helps us in many ways in our everyday life. Some of the applications are:
- Indigestion: When we suffer from acidity we take antacid to get relief. Antacid neutralises the effect of excessive acid.
- Ant bite: When an ant bites, moist baking soda solution or calamine solution is rubbed which neutralises the effect of acid injected into the skin when an ant bites.
- Soil treatment: Plants do not grow well when the soil is too acidic or too basic. When the soil is too acidic, it is treated with bases like quick lime or slaked lime and when it is basic, organic matter is added to it. Organic matter releases acids which neutralise the basic nature of the soil.
- Factory wastes: The factory wastes contain acids, they are harmful for water bodies. To neutralise these wastes, basic substances are added to them.
Question 3.
Fill in the crossword given with the help of the clues provided.
Across
(2) The solution which does not change the colour of either red or blue litmus.
(4) Phenolphthalein gives pink colour in this type of solution.
(7) Colour of blue litmus in lemon juice.
Down
(1) It is used to test whether a substance is acidic or basic.
(3) It is natural indicator and gives pink colour in basic solution.
(5) Nature of ant’s sting.
(6) It is responsible for increase in temperature during a neutralisation reaction.
Answer:
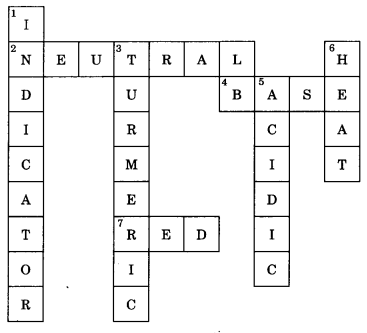
Acids, Bases and Salts Class 7 Extra Questions HOTS
Question 1.
If we add dilute sulphuric acid to lime water, what will happen to the reaction mixture?
Answer:
The reaction mixture will become hot due to neutralisation reaction between sulphuric acid and lime water.
Question 2.
In a reaction given below, how can we get solid sodium chloride from its solution?
Hydrochloric acid + Sodium hydroxide ➝ Sodium chloride + Water
Answer:
Through the process of evaporation we can get solid sodium chloride from its solution. We must evaporate the water in a container over a burner until all the water from the mixture evaporates.
Question 3.
Shweta took a little sodium hydroxide in a beaker and dipped a red litmus paper into it. She observed red litmus paper turned blue.
She took dilute hydrochloric acid in the same beaker and dipped a blue litmus paper into it. The blue litmus paper did not turn red. Why?
Answer:
Shweta took dilute hydrochloric acid in the same beaker containing sodium hydroxide solution. Mixing of base into acid forms salt which is neutral and does not change colour of either blue or red litmus paper.
Question 4.
Why litmus is better natural indicator than other natural indicator?
Answer:
Litmus give wide range of colour change from weak acid to strong acid and from weak base to strong base. Other natural indicator, on the other hand, do not show a wide colour change.
Question 5.
Is it advisable to drink lemonade during indigestion? Why?
Answer:
No it is not advisable to drink lemonade during indigestion because it is acidic in nature. Too much acidity in stomach causes indigestion. Drinking lemonade may worsen the condition.
Acids, Bases and Salts Class 7 Extra Questions Value Based (VBQs)
During performing an experiment on neutralisation reaction, Ravi took about 100 mL of sodium hydroxide solution and pour approximately the same amount of concentrated hydrochloric acid to it.
He observed a very vigorous reaction with evolution of large amount of heat. When he dipped litmus paper into the solution it turned red. He was surprised with the result though he had read all the instructions and precautions given to him by his teacher.
(a) What was the expected result of the experiment?
(b) What went wrong in the experiment performed by Ravi?
(c) Why Ravi observed a very vigorous reaction with evolution of large amount of heat when he added concentrated hydrochloric acid to sodium hydroxide?
(d) What value of Ravi is shown here?
Answer:
(a) It was expected that the solution form will be neutral and would not change colour of the litmus.
(b) Ravi added concentrated hydrochloric acid instead of dilute hydrochloric acid. This mistake can prove very dangerous.
(c) Hydrochloric acid is a strong acid and sodium hydroxide is a strong base themselves to produce enough heat when mixed together. In addition to this, Ravi used concentrated form of this acid which can even cause burns.
(d) Ravi showed carelessness over here by not reading the instructions and precautions carefully before performing an experiment.
Question 2.
Class VII students went for an educational trip to see how various industries and human settlements around river Yamuna, in Delhi, is polluting it deliberately. They saw that factory wastes and municipal wastes are dumped into it untreated. They thought of spreading an awareness programme. They even wrote to Municipal Corporation of the state suggesting ways to reduce this kind of pollution.
(a) How factory or domestic wastes affect the quality of river?
(b) How the process of neutralisation is effective in changing some of the quality of water?
(c) Suggest any two ways to reduce pollution of river Yamuna.
(d) Why is it important to check river pollution?
(e) What methods will you adopt to spread awareness against river pollution?
Answer:
(a) Factory or domestic waste changes colour, turbidity, acidity or basicity, temperature, microbial growth, etc., of the water.
(b) If the river water is too acidic or basic, it can affect the aquatic life. So by neutralising the pollutants before discharging into the river can at least change water quality to neutral.
(c) Pollution of river Yamuna can be reduced largely by making the people aware that how can they contribute in their own level to control water pollution and giving strict guidelines to industries situated near river bank to treat their waste suitably before discharging it into the river.
(d) It is important to check river pollution because they are our lifeline. We all are dependent mainly on river water for various activities like drinking water, irrigation, fishing, etc. Pollution of river water also affect the aquatic life.
(e) We will make poster, do some street dramas, will write an article in newspaper or social sites and most important will follow the rules to minimise water pollution by ourselves.